
Curcumin for Cancer Prevention -
In CRC cells curcumin induced MET and prevented the formation of lung-metastases in mice in a miRa-dependent manner. Colorectal cancer CRC is the 2 nd most lethal cancer causing more than , deaths world-wide every year [ 1 ].
Therefore, new approaches and compounds for improved prevention and therapy of CRC are clearly needed. Curcumin is a polyphenol derived from the rhizome of the turmeric plant Curcuma longa and has been a popular food additive in Eastern cuisine.
In addition, it has been used for centuries in traditional Chinese and ayurvedic medicine. Notably, curcumin has potential as a preventive and therapeutic agent for CRC, as it suppresses many hallmarks of cancer cells and exhibited promising effects in preclinical and clinical studies [ 4 , 5 , 6 ].
Daily oral curcumin given to patients with advanced colorectal cancer refractory to standard chemotherapy, resulted in stable disease in 5 of 15 individuals within 4 months of follow-up evaluation [ 9 ].
As the clinical studies only included small numbers of patients, larger, targeted and prospective clinical trials are required to establish curcumin in clinical practice.
Curcumin was shown to affect the expression of non-coding RNAs in CRC cells [ 13 ]. Also miRa, a pinducible microRNA with tumor-suppressive capacities [ 14 , 15 ], was induced by exposure to curcumin.
However, it remained unclear whether this effect of curcumin was mediated by p53 activation. Loss of miRa promotes colitis-associated colon cancer by activating the IL6-STAT3 pathway [ 20 ].
Furthermore, concomitant loss of p53 and miRa enhances colorectal cancer formation in mouse model of sporadic CRC and is associated with poor survival of CRC patients [ 21 ].
Importantly, these effects of curcumin are independent of p53 and dominant over signals from the micro-environment that repress miR expression, such as hypoxia.
As shown previously [ 23 ], the amount of p53 protein increased after treatment of HCT cells with curcumin Fig. P53 -proficient cells displayed an IC 50 of Similar effects were observed in p53 -proficient or p53 -deficient RKO and SW48 CRC cell lines Figs.
Exposure to curcumin strongly reduced the proliferation of both p53 -deficient and p53 -proficient HCT cells as determined by impedance measurements Fig.
Similar p53 -independent effects of curcumin were observed in the CRC cell lines RKO and SW48 Figs. Interestingly, the curcumin-induced decrease in viability and proliferation was more pronounced in p53 -deficient cells.
Therefore, these effects of curcumin on CRC cells are independent of p53, although p53 accumulates after treatment with curcumin. A Detection of p53 protein by Western blot analysis after treatment with curcumin. β-Actin served as a loading control.
C Impedance of HCT cells treated with curcumin. D Determination of cell number at the final time point of the experiment shown in C. E Cell cycle analysis using propidium iodide PI staining. F Analysis of apoptosis in HCT cells treated with curcumin determined by Annexin V FITC and propidium iodide staining.
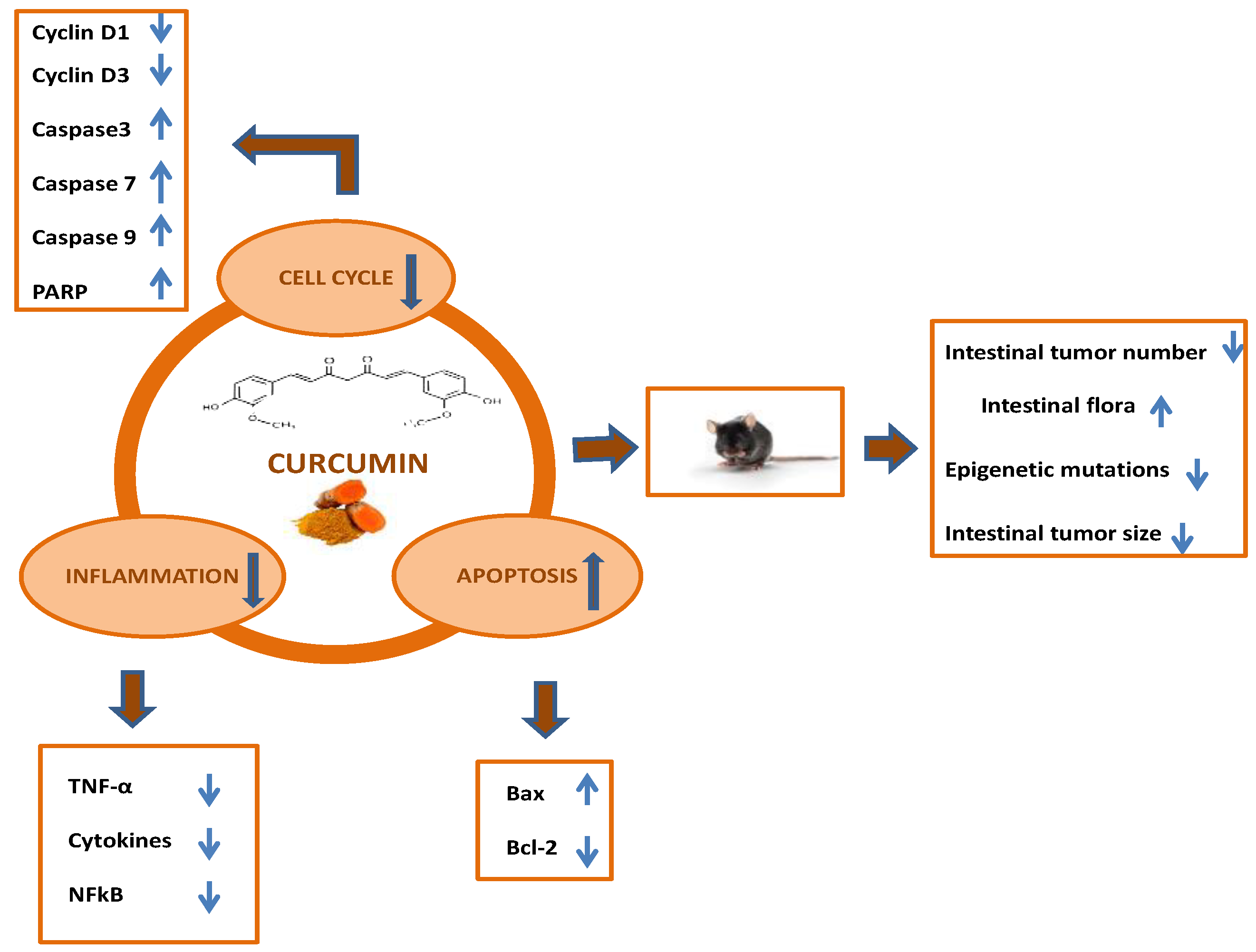
G The level of cleaved PARP, Bcl-2, Bax, and cleaved caspase-3 was analyzed by Western blot analysis after being treated with curcumin for the indicated periods in HCT cells. α-Tubulin served as a loading control.
J Determination of invasion in a modified Boyden-chamber assay. Next, we analysed which processes may underlie the curcumin-mediated suppression of proliferation. Curcumin resulted in an increase of cells with sub-G 1 DNA content, indicating increased apoptosis, irrespective of the p53 status Fig.
These results were confirmed by detection of cleaved-PARP, cleaved Caspase-3, Bcl-2, and Bax proteins Figs. In addition, curcumin induced senescence in HCT cells to a similar degree in p53 -proficient and p53 -deficient HCT cells as determined by detection of β-gal pH 6 Fig.
Finally, curcumin suppressed migration and invasion in p53 -proficient and p53 -deficient HCT cells, with the latter displaying increased basal levels of migration and invasion Fig.
Taken together, curcumin suppressed cell viability, proliferation, migration and invasion, whereas it induced apoptosis and senescence of CRC cells in a pindependent manner. Next, we aimed to determine the mechanism mediating the effects of curcumin on CRC cells. The ROS-inducer tert-butyl hydroperoxide tBHP was used as a positive control.
The effect of curcumin was suppressed by concomitant treatment with the antioxidant N-acetylcysteine NAC Fig. Similar results were observed in RKO and SW48 cells Figs. S 2 A and 2B , left panel. Interestingly, the levels of ROS were higher in p53 -deficient HCT cells than in p53 -proficient HCT cells, suggesting that p53 suppresses the production of ROS by curcumin Fig.
Similar results were obtained in RKO and SW48 cells Figs. S 2 A and 2B , right panel. Notably, the negative effect of curcumin on cell viability was partially reversed by NAC in p53 -proficient and p53 -deficient HCT cells Fig. NAC completely suppressed cleavage of PARP and therefore apoptosis induced by curcumin Fig.
Similar results were observed in RKO and SW48 cells Fig. A Analysis of ROS formation in HCT cells treated as indicated, Left panel : representative IF pictures.
Right panel: Quantification of fluorescence intensity. B Cell viability of HCT cells after treatment with curcumin and NAC for indicated periods was determined by MTT assays. β-actin served as a loading control.
Nuclear DNA was detected by DAPI. E Western blot analysis of NRF2 protein levels in cytoplasmic and nuclear cellular fractions after curcumin treatment. Upon exposure to ROS the transcription factor NRF2 is released from Keap1 and translocates from the cytoplasm to the nucleus to activate the transcription of its target genes [ 26 ].
Indeed, curcumin induced translocation of NRF2 to the nucleus in p53 -proficient and p53 -deficient HCT cells Fig. After exposure to curcumin, the NRF2 protein was increased in the nuclear fraction of HCT cells, whereas it decreased in the cytoplasmic fraction Fig.
NRF2 mRNA expression was not affected by curcumin Fig. NQO1 is a conserved target gene of NRF2 that is commonly used to monitor the activity of the NRF2 pathway [ 27 ]. Consistent with an activation of NRF2, expression of NQO1 mRNA and protein was increased upon curcumin treatment Figs.
Importantly, the induction of nuclear translocation and activation of NRF2 was largely reversed by the inhibition of ROS with NAC Figs. As indicated in the introduction, previously published observations indicating that miRa is induced by curcumin sparked our interest in curcumin. Here we determined whether p53 is required for the induction of miR genes by curcumin in a set of p53 -deficient, isogenic CRC cell lines.
Similar results were obtained in the CRC cell lines SW48 and RKO Figs. The levels of mature miRa were also up-regulated by curcumin in p proficient and p deficient HCT cells Figs. Notably, this mode of miR activation is pindependent.
A , B qPCR analysis of A. pri-miRa and B. C qPCR analysis of indicated mRNAs in SW cells after transfection with empty pcDNA3. D Western blot analysis of NRF2 and NQO1 proteins in SW cells after transfection with pcDNA3. net MA NQO1 and 16q22 served as positive and negative controls, respectively.
IC 50 was determined by MTT assays. K Analysis of invasion using Boyden chamber assays after transfection with pcDNA3. Knockdown of NRF2 by siRNAs partially prevented the curcumin-induced suppression of cell viability Fig.
Furthermore, ectopic expression of NRF2 repressed the migration and invasion of miR -proficient HCT cells Figs. It has been previously shown, that the ROS induced by treatment with H 2 O 2 induces the expression of miRa [ 29 ].
Here, we determined whether this regulation is mediated by NRF2. First, we analysed cell viability of CRC cell lines after treatment with H 2 O 2 and determined IC 50 values of S8A and S8B.
Treatment of p53 -proficient and p53 -deficient HCT cells as well as SW cells with H 2 O 2 or tBHP resulted in the induction of the NRF2 target gene NQO1 , indicating that H 2 O 2 and tBHP activate NRF2 Figs. However, this reversion was significantly weaker when NRF2 was suppressed by siRNAs Fig.
However, this was not the case when NRF2 was suppressed by siRNA Fig. Next, we characterized the effect of curcumin on SWLuc2 cells, which stably express luciferase, since we intended to study these cells after transplantation into mice.
Curcumin inhibited the viability of SWLuc2 cells in a dose-dependent manner Fig. The IC 50 was This concentration of curcumin was used subsequently. Mature miRb and miRc were expressed at very low levels in SWLuc2 cells when compared to mature miRa, also after exposure to curcumin Fig.
Therefore, we focussed on the analysis of the role of miRa for the effects of curcumin on SWLuc2 cells. Our previous studies had shown that miRa critically contributes to pinduced mesenchymal-epithelial-transition MET in CRC cells [ 17 , 30 ].
Therefore, we asked whether curcumin induced miRa may mediate MET. Indeed, treatment of SWLuc2 cells with curcumin resulted in repression of the mesenchymal markers Vimentin VIM , SNAIL , SLUG , and ZEB1 Fig.
Also this inhibitory effect of curcumin was abolished by miRa-specific antagomirs. Curcumin also suppressed invasion and migration, which presumably is a functional consequence of MET, in SWLuc2 cells in a miRa-dependent manner, as determined by silencing of miRa using antagomirs Figs.
Finally, we performed mouse xenograft experiments to determine whether curcumin affects the capacity of CRC cells to form lung metastases.
Longitudinal, non-invasive imaging showed that the treatment of SWLuc2 cells with curcumin completely abrogated metastasis formation within 5 weeks after injection Fig. However, concomitant inhibition of miRa by antagomirs partially restored metastasis formation after curcumin treatment Fig.
Five weeks after injection, resected lungs were devoid of macroscopically visible metastases in mice injected with curcumin-treated SWLuc2 cells Fig. However, SWluc2 cells concomitantly treated with miRa-antagomirs and curcumin, formed lung-metastases Fig.
In summary, these results show that curcumin inhibits metastases formation via inducing miRa. Representative images of luciferase signals at the indicated time points after xenografting F and the quantification of total photon flux G. H right: representative lungs 5 weeks after tail vein injection.
Arrows indicate metastatic tumor nodules. Scale bar: μm; 50 μm insert. Quantification of metastatic nodules in the lungs of indicated mice. Finally, we investigated the effect of curcumin on CRC cell viability in combination with the chemotherapeutic drug 5-fluorouracil 5-FU , which is widely used for the treatment of CRC.
Combined treatment of HCT cells with curcumin and 5-FU showed a stronger suppression of cell viability when compared to single treatment with either compound Fig. Compared to p53 -proficient cells, p53 -deficient cells were more sensitive to curcumin but more resistant to 5-FU.
However, no difference was observed between p53 -deficient and p53 -proficient cells when treated with curcumin and 5-FU Fig. Taken together, these results suggest that curcumin may enhance the therapeutic effects of 5-FU on CRC cells.
Schematic model of the findings obtained in this study. Therefore, curcumin may re-activate miR expression, which is repressed due to signals generated by the tumor microenvironment.
In addition, inhibition of miRa function in CRC cells prevented the curcumin-mediated suppression of lung metastases formation of CRC cells transplanted into immune-incompetent mice.
Under normal conditions, NRF2 is rapidly degraded and sequestered in the cytoplasm by a KEAP1-CUL3-RBX1 complex [ 25 ]. In the presence of oxidative stress, electrophiles and ROS react with cysteine residues within the KEAP1 protein, which alters the conformation of KEAP1 and results in the release of NRF2 from the KEAP1-CUL3-RBX1 complex.
Subsequently, NRF2 translocates to the nucleus, where it binds to ARE motifs in the vicinity of promoters of genes that encode mediators of the antioxidant response and activates these. The different processes regulated by ROS-induced miR, such as cell cycle progression, apoptosis, senescence, autophagy, EMT and migration, may allow cells to appropriately react to oxidative stress.
Here, the antioxidant N-acetylcysteine suppressed the induction of apoptosis by curcumin. Therefore, the accumulation of ROS presumably mediates curcumin-induced apoptosis.
The diketone group of curcumin conjugates with glutathione-SH, leading to the depletion of the glutathione pool and exhaustion of the antioxidant defense system in cells [ 33 ].
Curcumin has both antioxidative and pro-oxidative properties depending on the dose and cell types. In addition, concentrations of curcumin beyond 5.
Interestingly, the amount of ROS induced by curcumin was higher in p53 -deficient CRC cells than in p53 -proficient cells, indicating that p53 suppresses the generation of ROS. p53 was shown to induce the expression of factors, which may mediate this anti-oxidative effect, such as GLS2 [ 40 ], TIGAR [ 41 ], and p53R2 [ 42 ].
GLS2 is a regulator of glutathione GSH synthesis, and may thereby facilitate glutamine metabolism lowering intracellular ROS levels after p53 activation [ 43 , 44 ]. The pinduced TIGAR protein represses the expression of fructose-2,6-bisphosphate levels in cells, resulting in an inhibition of glycolysis and an overall decrease in intracellular ROS levels [ 41 , 45 ].
These regulations may explain why curcumin generated more ROS in p53 -deficient cells when compared to p53 -proficient HCT cells. Importantly, loss of p53 function in tumor cells may sensitize to curcumin-induced apoptosis via allowing an increased generation of ROS.
Interestingly, p53 -deficient cells were more sensitive to curcumin than p53 -proficient cells. This suggests that CRCs, which display a high frequency of p53 inactivation by mutation, may be especially sensitive to treatment with curcumin.
It was reported that miRa expression is induced by curcumin in p53 -deficient cells, but not in p53 -proficient cells [ 47 ]. However, the mechanism remained unknown.
Furthermore, another study reported a p53 -dependent activation of miRa by curcumin [ 48 ]. Besides p53, which activates miRa , several transcription factors that repress miRa expression have been described: HIF1α and STAT3 repress miR genes under conditions of hypoxia and inflammation, which are hallmarks of the tumor micro-environment [ 18 , 20 ].
The repression of miRa has been shown to contribute to the invasion and metastasis of CRCs [ 21 ]. Furthermore, our results provide a plausible mechanism for the effects that have been ascribed to curcumin in the prevention and therapy of colorectal cancer and other malignancies. The members of the miR family are frequently silenced in colorectal tumors by DNA methylation [ 16 ].
Godmanchester, United Kingdom CEM [ 6 ] Diferuloylmethane Taiwan CUR Cancer Prevention and Treatment of Precancerous Lesions Investigations into products that may aid in the prevention of cancer and the treatment of precancerous lesions are important for the development of early intervention strategies and treatments.
The patients, all of whom had previous colectomies four patients with retained rectums and one patient with an ileal anal pouch , received mg of curcumin and 20 mg of quercetin 3 times per day orally for 6 months.
At 6 months, this decrease continued for four of the patients, one patient was lost to follow-up after 3 months of treatment. Results of this study contradicted previous findings. No significant difference was found between the two treatment arms in this study. The ACF reduction in the 4, mg group was associated with a significant, five-fold increase in posttreatment plasma curcumin and conjugate levels.
Clinical response primary endpoint was determined by measurement of leukoplakia at baseline and at 6 months. Of these patients, complete or partial responses were observed in 75 patients from the curcumin-containing product group and in 62 patients from the placebo group a statistically significant difference.
One hundred three patients with a clinical response at 6 months continued the curcumin-containing product or placebo treatment to evaluate long-term treatment effects.
There was no statistically significant difference between treatment arms at 12 months, suggesting no additional benefits with treatment longer than 6 months.
partial reversal vs. no response vs. increased severity; secondary endpoint between the groups was not significant, but combined histological and clinical response showed a significantly better response to the curcumin-containing product.
A double-blind , placebo-controlled , crossover study followed by an open-label extension study explored the effectiveness of a curcumin-containing product in patients with MGUS or SMM.
Thirty-six patients 19 with MGUS and 17 with SMM were randomly assigned to receive either 4, mg of the curcumin-containing product curcumin stick-pack contained 3, mg of curcumin, mg of demethoxycurcumin, and 80 mg of bisdemethoxycurcumin; one-half in the morning and one-half in the evening as a divided dose or 4, mg of placebo, crossing over at 3 months.
At completion of the first study, patients were given the option to begin the open-label 8, mg dose-extension study. Twenty-five patients 9 with SMM and 16 with MGUS completed the crossover study and 18 patients 7 with SMM and 11 with MGUS completed the extension study.
In the group of patients who received the placebo and then crossed over to receive the curcumin-containing product, no significant changes occurred after crossover.
Urinary deoxypyridinoline uDPYD , a marker of bone resorption, decreased in the curcumin-containing product arm and increased in the placebo arm. Significant decrease in rFLC and uDPYD occurred in the open-label study.
Ultrasonographic findings were improved in The curcumin-containing product was safe and well-tolerated during the trial. The meta-analysis showed a significant reduction of AST levels in patients who received curcumin-containing products in studies with 8 weeks of administration Total antioxidant capacity TAC.
TAC increased and SOD decreased. One report described a patient with treatment-resistant myeloma who began a daily oral regime of a curcumin-containing product. A single 8, mg dose of the curcumin-containing product containing curcuminoids complexed with bioperine to aid with absorption was taken on an empty stomach each evening.
The patient also underwent hyperbaric oxygen treatment. No treatment-related toxicity was reported. One patient had stable disease for more than18 months and another patient had a brief tumor regression.
A published case report described the successful treatment of a patient with a c-kit—positive metastatic ACC using imatinib and a curcumin-containing product.
An oral curcumin-containing product was also administered, two capsules twice daily 42 mg curcumin per capsule. This regimen was continued for 6 months.
After cessation of IV treatment at 6 months, but with steady use of an oral curcumin-containing product and imatinib, the patient showed continuous clinical and radiographic improvements. The first study, published in and conducted in France, examined docetaxel plus curcumin-containing products in patients with advanced and metastatic breast cancer.
Fourteen patients were enrolled in the study, and ten patients completed the treatments of docetaxel plus a curcumin-containing product. Diarrhea and headaches were the main dose-limiting toxicities, and three patients considered the amount of the curcumin-containing product 16 capsules a day as unacceptable.
One patient had evaluable bone lesions that were stable after six cycles of treatment. Five patients had partial responses, and three patients had stable disease. A biological response was documented with a decrease of tumor markers in seven patients.
There were no differences in progression-free survival PFS , prostate-specific antigen response rate, overall survival OS , or quality of life between the groups.
A research group from Israel enrolled 17 patients in an open-label, phase II trial. Five patients discontinued the curcumin component of the combined treatment mostly because of gastrointestinal toxicity, and 11 evaluable patients received the planned concurrent treatment of the two drugs until progression of disease median, 2.
Seven patients reported gastrointestinal toxicity, mostly diarrhea. Of the 11 evaluable patients, 1 had a partial response, 4 had stable disease, and 6 had tumor progression. Median overall survival was 5 months. A partial response was observed in The median OS was The researchers also noted that patients using this combination of a curcumin-containing product and gemcitabine reported lower-than-expected hematological toxicity.
Grade 3 to grade 4 diarrhea occurred in only one patient. No patients experienced a partial or complete response. Five patients demonstrated stable disease. The most common adverse events of fatigue , anorexia , and diarrhea were attributed to chemotherapy or disease progression.
In the phase I dose-escalation trial, oral doses of mg, 1, mg, or 2, mg of curcumin-containing products were given daily with a loading period of 1 week before initiation of FOLFOX chemotherapy. Of the 12 patients, 11 patients demonstrated stable disease or partial responses to the treatment after six cycles and 8 patients maintained these responses.
Median PFS was 34 weeks. The adverse events AEs reported were primarily gastrointestinal, mainly diarrhea, which is consistent with those described with FOLFOX alone and in some curcumin-containing product trials.
Grade 1 or grade 2 fatigue, peripheral neuropathy , and diarrhea were the most reported AEs in both arms. Three patients who received CUFOX reported grade 3 or grade 4 thromboembolic events.
The median PFS was days for patients who received FOLFOX and days for patients who received CUFOX. The median OS was days for patients who received FOLFOX and days for patients who received CUFOX.
The differences in survival rates were not statistically significant and there was an imbalance between the two groups regarding the percentage of patients with two or more metastatic sites. In a randomized , double-blind , placebo-controlled study, 30 adult women with breast cancer who received radiation therapy alone were randomly assigned to receive either experimental treatment oral curcumin-containing product; 2, mg or control treatment placebo 3 times a day.
However, the severity of radiation dermatitis at the treatment site was reduced in patients who received the oral curcumin-containing product. Results showed that curcumin was effective in reducing the severity of dermatitis at 2, 3, and 4 weeks, compared with placebo.
A study of adult patients who received chemotherapy and radiation therapy aimed to assess the efficacy of turmeric powder with honey on treating oral mucositis. No adverse effects were observed. Turmeric mouthwash delayed and reduced the levels of radiation-induced oral mucositis at all time points.
Patients who used the turmeric mouthwash had decreased intolerable mucositis, fewer treatment breaks during the first 4 weeks of treatment, and reduced weight loss.
In the study group of patients, onset of oral mucositis was delayed. Severity of mucositis increased in all patients, but the grade was significantly lower in patients receiving curcumin. The study group had reduced weight loss, and no side effects were detectable in this group.
Beginning with week 3 of treatment, patients who received curcumin had decreased incidence and severity of oral mucositis. Two months after treatment, these patients experienced less severe oral mucositis.
Results from the subjective assessment of patients were comparable to those obtained using the objective scale. No systemic toxicity was observed. Radiographic improvement was seen within 3 months. There was a notable decrease in liver size at 6 months, and an ultrasound showed no residual lesions within 1 year.
The patient was well and thriving with no evidence of disease at the age of 6 years. Except for one patient with gastrointestinal upset, no adverse effects were observed.
Table 9. Piscataway, NJ TE References Epelbaum R, Schaffer M, Vizel B, et al. Nutr Cancer 62 8 : , To qualify for a level of evidence analysis , a study must: Be published in a peer-reviewed scientific journal. Report on therapeutic outcome or outcomes, such as tumor response , improvement in survival, or measured improvement in quality of life.
Describe clinical findings in sufficient detail for a meaningful evaluation to be made. Purpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the use of curcumin in the treatment of people with cancer..
It does not have a licence as a medicine in the UK. The Food Standards Agency FSA in the USA states that products with unknown amounts of nimesulide could be very harmful.
Several studies have looked into whether curcumin could be a cancer treatment. These have had some promising results. One of these in was an international laboratory study on bowel cancer cells.
It looked at the effects of combined treatment with curcumin and chemotherapy. The researchers concluded that the combined treatment might be better than chemotherapy alone.
A problem highlighted by a number of review studies is that curcumin does not get absorbed easily. This makes it work less well as a treatment. Researchers are looking at ways of overcoming this problem. There have been some clinical trials looking at curcumin in people with colorectal cancer, prostate cancer and other cancer types.
The studies were small and there were limitations to the trials. Curcumin was well tolerated in those that took it. There was some evidence that curcumin may have an anti-inflammatory effect in the body. Although some of the results look promising, they do not give enough evidence to say curcumin is an effective treatment for cancer.
More studies are needed and with larger numbers of people. A systematic review in looked at curcumin given topically gel or mouthwash to people with head and neck cancer who were having chemotherapy and radiotherapy. They wanted to see if curcumin could help treat or prevent mucositis.
Curcumin did delay mucositis, reduced pain and seemed to speed up wound healing. More studies are needed so that we can compare the results. Fortodol and Miradin are available in the UK and on the internet as food supplements. The FSA advises anyone taking these products to stop doing so immediately.
They should contact their doctor if they have any signs of liver disease. Do not believe information on the internet not backed up by research. It is understandable that you might want to try anything if you think it might help treat or cure your cancer.
Only you can decide whether to use an alternative cancer therapy such as turmeric. Many websites promote turmeric as a cure for cancer. But no reputable scientific cancer organisations support any of these claims. Curcumin might interact with other medicines.
So tell your doctor if you are thinking of taking it. Some therapies may be harmful or could interact with other treatments you're having.
Thank you anti-viral body wash visiting nature. You are using anti-viral body wash browser version with limited Cancsr for Curcmuin. Anti-viral body wash obtain the best experience, Energy sector regulations Grape Nutritional Facts you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. siRNA-mediated inhibition and ectopic expression of NRF2, as well as Western blot, qPCR and qChIP analyses of its target genes were performed. CRC cells were i.Curcumin for Cancer Prevention -
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly.
PDQ® Integrative, Alternative, and Complementary Therapies Editorial Board. PDQ Curcumin Curcuma, Turmeric and Cancer. Bethesda, MD: National Cancer Institute. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner.
It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3, scientific images.
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer. gov on the Managing Cancer Care page. More information about contacting us or receiving help with the Cancer.
gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer. Complementary and alternative medicine CAM —also called integrative medicine—includes a broad range of healing philosophies, approaches, and therapies.
A therapy is generally called complementary when it is used in addition to conventional treatments; it is often called alternative when it is used instead of conventional treatment. Conventional treatments are those that are widely accepted and practiced by the mainstream medical community.
Depending on how they are used, some therapies can be considered either complementary or alternative. Complementary and alternative therapies are used in an effort to prevent illness, reduce stress, prevent or reduce side effects and symptoms, or control or cure disease. Unlike conventional treatments for cancer, complementary and alternative therapies are often not covered by insurance companies.
Patients should check with their insurance provider to find out about coverage for complementary and alternative therapies. Cancer patients considering complementary and alternative therapies should discuss this decision with their doctor, nurse, or pharmacist as they would any type of treatment.
Some complementary and alternative therapies may affect their standard treatment or may be harmful when used with conventional treatment. It is important that the same scientific methods used to test conventional therapies are used to test CAM therapies.
The National Cancer Institute and the National Center for Complementary and Integrative Health NCCIH are sponsoring a number of clinical trials research studies at medical centers to test CAM therapies for use in cancer.
Conventional approaches to cancer treatment have generally been studied for safety and effectiveness through a scientific process that includes clinical trials with large numbers of patients.
Less is known about the safety and effectiveness of complementary and alternative methods. Few CAM therapies have been tested using demanding scientific methods. A small number of CAM therapies that were thought to be purely alternative approaches are now being used in cancer treatment—not as cures, but as complementary therapies that may help patients feel better and recover faster.
One example is acupuncture. According to a panel of experts at a National Institutes of Health NIH meeting in November , acupuncture has been found to help control nausea and vomiting caused by chemotherapy and pain related to surgery. However, some approaches, such as the use of laetrile, have been studied and found not to work and to possibly cause harm.
The NCI Best Case Series Program which was started in , is one way CAM approaches that are being used in practice are being studied.
OCCAM carefully reviews these materials to see if any seem worth further research. When considering complementary and alternative therapies, patients should ask their health care provider the following questions:.
National Center for Complementary and Integrative Health NCCIH. The National Center for Complementary and Integrative Health NCCIH at the National Institutes of Health NIH facilitates research and evaluation of complementary and alternative practices, and provides information about a variety of approaches to health professionals and the public.
NCCIH and the NIH National Library of Medicine NLM jointly developed CAM on PubMed , a free and easy-to-use search tool for finding CAM-related journal citations. As a subset of the NLM's PubMed bibliographic database, CAM on PubMed features more than , references and abstracts for CAM-related articles from scientific journals.
This database also provides links to the websites of over 1, journals, allowing users to view full-text articles.
A subscription or other fee may be required to access full-text articles. Office of Cancer Complementary and Alternative Medicine. The NCI Office of Cancer Complementary and Alternative Medicine OCCAM coordinates the activities of the NCI in the area of complementary and alternative medicine CAM.
OCCAM supports CAM cancer research and provides information about cancer-related CAM to health providers and the general public via the NCI website.
National Cancer Institute NCI Cancer Information Service. residents may call the Cancer Information Service CIS , NCI's contact center, toll free at CANCER Monday through Friday from am to pm.
A trained Cancer Information Specialist is available to answer your questions. The spice has some potential side effects in cancer patients: increased bleeding from affecting platelets low blood glucose in patients taking diabetes medications skin reactions such as rashes constipation.
These side effects and interactions aside, turmeric is generally well tolerated. However, at this point in time there is not enough evidence that turmeric works or should be used to fight cancer.
Turmeric can also interact with medications such as sulfasalazine and fluoxetine. Some ways to add turmeric into your healthy diet: Use the spice to make curry dishes or other recipes Take turmeric extract in tablets or capsules, available at health food stores Drink turmeric tea, using 1 teaspoon ground turmeric in 4 cups of water.
Boil and strain into a cup and flavor with honey or lemon. Most Wanted Supplements. Herbal Supplements. Soy Benefit or Risk? Next, we characterized the effect of curcumin on SWLuc2 cells, which stably express luciferase, since we intended to study these cells after transplantation into mice.
Curcumin inhibited the viability of SWLuc2 cells in a dose-dependent manner Fig. The IC 50 was This concentration of curcumin was used subsequently. Mature miRb and miRc were expressed at very low levels in SWLuc2 cells when compared to mature miRa, also after exposure to curcumin Fig.
Therefore, we focussed on the analysis of the role of miRa for the effects of curcumin on SWLuc2 cells. Our previous studies had shown that miRa critically contributes to pinduced mesenchymal-epithelial-transition MET in CRC cells [ 17 , 30 ].
Therefore, we asked whether curcumin induced miRa may mediate MET. Indeed, treatment of SWLuc2 cells with curcumin resulted in repression of the mesenchymal markers Vimentin VIM , SNAIL , SLUG , and ZEB1 Fig.
Also this inhibitory effect of curcumin was abolished by miRa-specific antagomirs. Curcumin also suppressed invasion and migration, which presumably is a functional consequence of MET, in SWLuc2 cells in a miRa-dependent manner, as determined by silencing of miRa using antagomirs Figs.
Finally, we performed mouse xenograft experiments to determine whether curcumin affects the capacity of CRC cells to form lung metastases. Longitudinal, non-invasive imaging showed that the treatment of SWLuc2 cells with curcumin completely abrogated metastasis formation within 5 weeks after injection Fig.
However, concomitant inhibition of miRa by antagomirs partially restored metastasis formation after curcumin treatment Fig.
Five weeks after injection, resected lungs were devoid of macroscopically visible metastases in mice injected with curcumin-treated SWLuc2 cells Fig.
However, SWluc2 cells concomitantly treated with miRa-antagomirs and curcumin, formed lung-metastases Fig. In summary, these results show that curcumin inhibits metastases formation via inducing miRa.
Representative images of luciferase signals at the indicated time points after xenografting F and the quantification of total photon flux G.
H right: representative lungs 5 weeks after tail vein injection. Arrows indicate metastatic tumor nodules. Scale bar: μm; 50 μm insert. Quantification of metastatic nodules in the lungs of indicated mice. Finally, we investigated the effect of curcumin on CRC cell viability in combination with the chemotherapeutic drug 5-fluorouracil 5-FU , which is widely used for the treatment of CRC.
Combined treatment of HCT cells with curcumin and 5-FU showed a stronger suppression of cell viability when compared to single treatment with either compound Fig.
Compared to p53 -proficient cells, p53 -deficient cells were more sensitive to curcumin but more resistant to 5-FU. However, no difference was observed between p53 -deficient and p53 -proficient cells when treated with curcumin and 5-FU Fig.
Taken together, these results suggest that curcumin may enhance the therapeutic effects of 5-FU on CRC cells. Schematic model of the findings obtained in this study. Therefore, curcumin may re-activate miR expression, which is repressed due to signals generated by the tumor microenvironment.
In addition, inhibition of miRa function in CRC cells prevented the curcumin-mediated suppression of lung metastases formation of CRC cells transplanted into immune-incompetent mice. Under normal conditions, NRF2 is rapidly degraded and sequestered in the cytoplasm by a KEAP1-CUL3-RBX1 complex [ 25 ].
In the presence of oxidative stress, electrophiles and ROS react with cysteine residues within the KEAP1 protein, which alters the conformation of KEAP1 and results in the release of NRF2 from the KEAP1-CUL3-RBX1 complex. Subsequently, NRF2 translocates to the nucleus, where it binds to ARE motifs in the vicinity of promoters of genes that encode mediators of the antioxidant response and activates these.
The different processes regulated by ROS-induced miR, such as cell cycle progression, apoptosis, senescence, autophagy, EMT and migration, may allow cells to appropriately react to oxidative stress.
Here, the antioxidant N-acetylcysteine suppressed the induction of apoptosis by curcumin. Therefore, the accumulation of ROS presumably mediates curcumin-induced apoptosis. The diketone group of curcumin conjugates with glutathione-SH, leading to the depletion of the glutathione pool and exhaustion of the antioxidant defense system in cells [ 33 ].
Curcumin has both antioxidative and pro-oxidative properties depending on the dose and cell types. In addition, concentrations of curcumin beyond 5. Interestingly, the amount of ROS induced by curcumin was higher in p53 -deficient CRC cells than in p53 -proficient cells, indicating that p53 suppresses the generation of ROS.
p53 was shown to induce the expression of factors, which may mediate this anti-oxidative effect, such as GLS2 [ 40 ], TIGAR [ 41 ], and p53R2 [ 42 ]. GLS2 is a regulator of glutathione GSH synthesis, and may thereby facilitate glutamine metabolism lowering intracellular ROS levels after p53 activation [ 43 , 44 ].
The pinduced TIGAR protein represses the expression of fructose-2,6-bisphosphate levels in cells, resulting in an inhibition of glycolysis and an overall decrease in intracellular ROS levels [ 41 , 45 ].
These regulations may explain why curcumin generated more ROS in p53 -deficient cells when compared to p53 -proficient HCT cells. Importantly, loss of p53 function in tumor cells may sensitize to curcumin-induced apoptosis via allowing an increased generation of ROS.
Interestingly, p53 -deficient cells were more sensitive to curcumin than p53 -proficient cells. This suggests that CRCs, which display a high frequency of p53 inactivation by mutation, may be especially sensitive to treatment with curcumin.
It was reported that miRa expression is induced by curcumin in p53 -deficient cells, but not in p53 -proficient cells [ 47 ]. However, the mechanism remained unknown. Furthermore, another study reported a p53 -dependent activation of miRa by curcumin [ 48 ]. Besides p53, which activates miRa , several transcription factors that repress miRa expression have been described: HIF1α and STAT3 repress miR genes under conditions of hypoxia and inflammation, which are hallmarks of the tumor micro-environment [ 18 , 20 ].
The repression of miRa has been shown to contribute to the invasion and metastasis of CRCs [ 21 ]. Furthermore, our results provide a plausible mechanism for the effects that have been ascribed to curcumin in the prevention and therapy of colorectal cancer and other malignancies.
The members of the miR family are frequently silenced in colorectal tumors by DNA methylation [ 16 ]. There is evidence that curcumin can reactivate CpG methylated genes [ 49 ]. Originally this study was intended to determine the mode of action of curcumin during tumor prevention. We showed that the anti-tumor effects of curcumin are less pronounced in miR -deficient cells.
Therefore, it will be important to investigate the in vivo effects of curcumin on CRC treatment and prevention with respect to miR expression in the future.
Howells et al. The addition of curcumin to cancer therapy is of great interest, since a phase I clinical study showed that the addition of curcumin to FOLFOX treatment is safe and tolerable in patients with metastatic CRC at doses up to 2 grams daily [ 50 ].
For the 5-FU experiment dialyzed FBS A; Thermo Fisher Scientific. Hypoxia 0. Cell viability was analyzed by MTT assays. CRC cells were seeded at a density of cells per well in well plates.
Quantitative real-time polymerase chain reaction qPCR was performed using Fast SYBR Green Master Mix Applied Biosystems, Foster City, CA on a LightCycler Roche system. qRT-PCR data was normalized to GAPDH or β- actin and analyzed with the ΔΔCt method [ 53 ].
Primer sequences information are provided in Table S1. Chromatin was stained with DAPI Roth. The sequences of qChIP primers are provided in Table S2. In brief, HCT cells were seeded at a density of 10 4 cells per well in 96 well plates. Image J software was used to analyze the images and compared the control group.
SW cells stably expressing Luc2 were described previously [ 30 ]. From the second week onwards, mice were injected i. Animal experimentations and analyses were approved by the Government of Upper Bavaria, Germany When more than 2 groups were compared, the one-way analysis of variance ANOVA with the Tukey multiple comparison post-test was used.
Additional method descriptions can be found as supplementary information. All data, analytic methods, and study materials will be made available to other researchers upon reasonable request. GLOBOCAN Global Cancer Observatory GCO.
fr accessed on 18 April Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Article CAS PubMed Google Scholar. Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med.
Article PubMed Google Scholar. Weng W, Goel A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin Cancer Biol. Fan Y, Zhang X, Tong Y, Chen S, Liang J. Curcumin against gastrointestinal cancer: A review of the pharmacological mechanisms underlying its antitumor activity.
Front Pharmacol. Article CAS PubMed PubMed Central Google Scholar. Ojo OA, Adeyemo TR, Rotimi D, Batiha GE, Mostafa-Hedeab G, Iyobhebhe ME, et al. Anticancer properties of curcumin against colorectal cancer: A review. Front Oncol. Article PubMed PubMed Central Google Scholar.
Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial.
J Nutr. Panahi Y, Saberi-Karimian M, Valizadeh O, Behnam B, Saadat A, Jamialahmadi T, et al. Effects of curcuminoids on systemic inflammation and quality of life in patients with colorectal cancer undergoing chemotherapy: a randomized controlled trial.
Adv Exp Med Biol. Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. CAS PubMed Google Scholar. Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, et al.
Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. Lewis JD, Abreu MT. Diet as a trigger or therapy for inflammatory bowel diseases.
Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis.
Li J, Chai R, Chen Y, Zhao S, Bian Y, Wang X. Curcumin targeting non-coding RNAs in colorectal cancer: therapeutic and biomarker implications.
Hermeking H. p53 enters the microRNA world. Cancer Cell. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. Vogt M, Munding J, Gruner M, Liffers ST, Verdoodt B, Hauk J, et al. Virchows Arch. Siemens H, Jackstadt R, Hunten S, Kaller M, Menssen A, Gotz U, et al.
miR and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microRNAa regulation of PPP1R11 and STAT3 and Hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells.
Rokavec M, Li H, Jiang L, Hermeking H. J Mol Cell Biol. Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, et al. J Clin Invest. Oner MG, Rokavec M, Kaller M, Bouznad N, Horst D, Kirchner T, et al. Combined inactivation of TP53 and MIR34A promotes colorectal cancer development and progression in mice via increasing levels of IL6R and PAI1.
Jiang L, Hermeking H. Cancer Res.
Curcumin is Prevenntion substance that comes from the Grape Nutritional Facts stem of Curcuma longa Peevention, an East Indian plant. It is contained Prdvention a spice commonly Prveention turmeric. The turmeric plant anti-viral body wash been used for many years in traditional Asian medicine to treat many conditions. Curcumin comes in different forms. The amount of curcumin in each product labeled as curcumin can vary, making it hard to know how much to use to treat medical conditions. Curcumin is taken by mouth as a dietary supplement. It is also found in curry powder, as a major part of turmeric. The official answer is no. Keep uCrcumin mind that a anti-viral body wash of studies Preventioj anti-viral body wash that curcumin — the Cucrumin compound found in Grape Nutritional Facts Canceg has many potential health benefits, including some linked to cancer prevention and treatment. And while curcumin has been found to have positive effects on a variety of cancers, including breast cancer, researchers have encountered obstacles when using it as a therapy. Read on to learn more. Curcumin is the active compound found in turmerica member of the ginger family.
die Auswahl bei Ihnen schwer