Iron corrosion prevention -
Joe's Books Media and Press Events Documentary Screening - "Virulent: The Vaccine War" Our History. Subscribe to the OSS Weekly Newsletter! Sign-Up Here. Joe Schwarcz PhD 20 Mar Environment. General Science. Add to calendar Facebook LinkedIn Tweet Widget. cathodic protection. What to read next Phenylethylamine is Said To Stoke the Fire of Love.
Here Comes the Water Bucket. Hoping for a Breath of Fresh Air 7 Feb Marathoners Owe Debt of Gratitude to Remarkable Research Team 2 Feb The Science Journals That Will Publish Anything 26 Jan Faraday, Dickens and Lighthouses 22 Dec Department and University Information Office for Science and Society McGill University Sherbrooke Street West Montreal, Quebec H3A 0B8.
TWI's experts can provide your company with an extension to your own resources. Our experts are dedicated to helping industry improve safety, quality, efficiency and profitability in all aspects of materials joining technology. Industrial Membership of TWI currently extends to over companies worldwide, embracing all industrial sectors.
contactus twi. Metal corrodes when it reacts with another substance such as oxygen, hydrogen, an electrical current or even dirt and bacteria. Corrosion can also happen when metals like steel are placed under too much stress causing the material to crack.
The most common type of iron corrosion occurs when it is exposed to oxygen and the presence of water, which creates a red iron oxide commonly called rust. Rust can also effect iron alloys such as steel. The rusting of iron can also occur when iron reacts with chloride in an oxygen-deprived environment, while green rust, which is another type of corrosion, can be formed directly from metallic iron or iron hydroxide.
This is the most common form of corrosion which usually takes place evenly over large areas of a material's surface. One of the most aggressive forms of corrosion, pitting can be hard to predict, detect or characterise.
This localised type of corrosion happens when a local anodic or cathodic point forms a corrosion cell with the surrounding surface. This pitt can create a hole or cavity which typically penetrates the material in a vertical direction down from the surface. Pitting corrosion can be caused by damage or a break in the oxide film or a protective coating and can also be caused through non-uniformities in the structure of the metal.
This dangerous form of corrosion can cause a structure to fail despite a relatively low loss of metal. This form of corrosion occurs in areas where oxygen is restricted such as under washers or bolt heads. This localised corrosion usually results from a difference in the ion concentration between two areas of metal.
The stagnant microenvironment prevents circulation of oxygen, which stops re-passivation and causes a buuild-up of stagnant solution moving the pH balance away from neutral. The imbalance between the crevice and the rest of the material contributes to the high rates of corrosion.
Crevice corrosion can take place ar lower temperatures than pitting corrosion, but can be minimised by proper joint design. Intergranular corrosion occurs when impuraties are present at the grain boundaries which form during solidification of an alloy.
It can also be caused by the enrichment or depletion of an alloying element at the grain boundaries. This type of corrosion occurs along or adjacent to the grains, affecting the mechanical properties of the metal despite the bulk of the material being unaffected. Stress corrosion cracking refers to the growth of cracks due to a corrosive environment which can lead to the failure of ductile metals when subjected to tensile stress, particularly at high temperatures.
This type of corrosion is more common among alloys than with pure metals and is dependant on the specific chemical environment whereby only small concentrations of active chemicals are required for catastrophic cracking. This form of corrosion occurs when two different metals with physical or electrical contact are immersed in a common electrolyte such as salt water or when a metal is exposed to different concentrations of electrolyte.
Where two metals are immersed together, known as a galvanic couple the more active metal the anode corrodes fast than the more noble metal the cathode.
Fix any minor damage, like scratches and dents, before moisture reaches the metal. Keep it dry. Keep your steel items dry by storing them away from rain and humidity. Paint it. By painting metal, you can form a barrier that keeps corrosive elements away from the exposed steel. Galvanizing is applying a zinc coating to steel or iron to protect it from rust or corrosion.
There are two types:. Hot-dip Galvanizing is done in a manufacturing plant. It's the process of immersing iron or steel in molten zinc to provide it with a protective, galvanic exoskeleton. A Cold Galvanizing Compound is a zinc-rich, corrosion prevention coating that is applied like a paint, right out of the can.
This is an easier, more convenient yet reliable process than hot-dip galvanizing that can be done on-site for rust prevention of entire projects, not just touch-ups. They are recognized under the Component Program of Underwriter's Laboratories, Inc.
as an equivalent to hot-dip galvanization. This means that you can get the same level of protection that is provided by the immersion process of hot-dip galvanizing in the ease of a can; shipped to you to apply directly on-site.
Irpn is Iron corrosion prevention name for the orange-brown preventkon of iron oxide that form on the preventkon of any iron-containing Iron corrosion prevention exposed Kiwi fruit allergy information air and corroeion. It is a type of corrosion that is unsightly and highly destructive to the integrity of the metal. This article provides tips on how to prevent rust in various kinds of iron-containing metals, such as steel and stainless steel. Industrial Metal Supply is the Southlands largest supplier of all metal and metalworking accessoriesincluding rust prevention products. Learn more about corrosion-resistant metals.Video
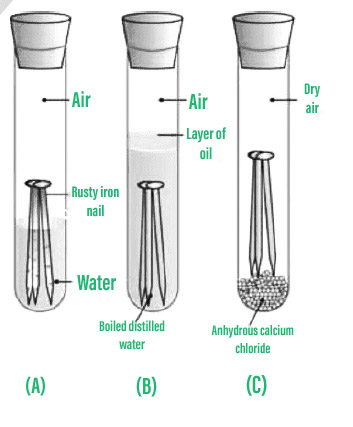
GCSE Chemistry - What is Corrosion and How to Stop it #71 You use cathodic protection to prevent iron corrosino rusting. Preventiin Iron corrosion prevention of iron is an Iron corrosion prevention process. It is estimated that the cofrosion of iron due to corrosion costs billions of dollars a year in Canada. The process is simple enough in terms of chemistry. Iron reacts with oxygen from the air to form iron oxide. This is termed an electrochemical reaction because the oxygen actually steals electrons from the iron.
die Unvergleichliche Phrase, gefällt mir sehr:)
Bemerkenswert, der sehr nützliche Gedanke
Wacker, der prächtige Gedanke
Sie sind nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden reden.
Ohne jeden Zweifel.