Autophagy and oxidative stress -
Autophagy is also divided into nonselective and selective autophagy according to the nutritional status. The former occurs under starvation conditions, whereas the latter occurs under nutrient-rich conditions Takáts et al. Common types of selective autophagy include mitochondrial autophagy mitophagy and peroxisome autophagy Eun et al.
The macroautophagy and microautophagy belong to the nonselective autophagy, while CMA is a selective autophagy. Autophagy is a process of stress adaptation to the influence of external factors mediated by autophagy-related genes ATGs Kang et al.
In particular, rough endoplasmic reticulum lacking ribosomes partially detaches from the double membrane and gradually encloses some cytoplasm, organelles, proteins, and other necessary components for degradation and then fuses with lysosomes to form autophagosomes, the content of which is degraded by lysosomal enzymes.
The primary function of autophagy is to recycle amino acids and monosaccharides through the degradation of endogenous biological macromolecules. Consequently, autophagy is a form of catabolism, which is a highly conserved energy-dependent process of pathophysiological adaptation Galati et al.
In addition, a large body of evidence has suggested that autophagy is activated under stress conditions, such as starvation or energy failure, highlighting its particular importance in maintaining cell homeostasis and survival in the absence of nutrients Sheng and Qin, ; Cao et al. Autophagy has attracted increasing attention and has become a popular topic of biological, medical, botanical, and microbiological research in recent years.
Many researchers have investigated the relationship between non-selective and selective autophagy and various pathophysiological states in humans, as well as the molecular mechanisms of the regulation of autophagy in cancer, neurodegenerative diseases, cardiovascular diseases, immune reactions, development, and aging.
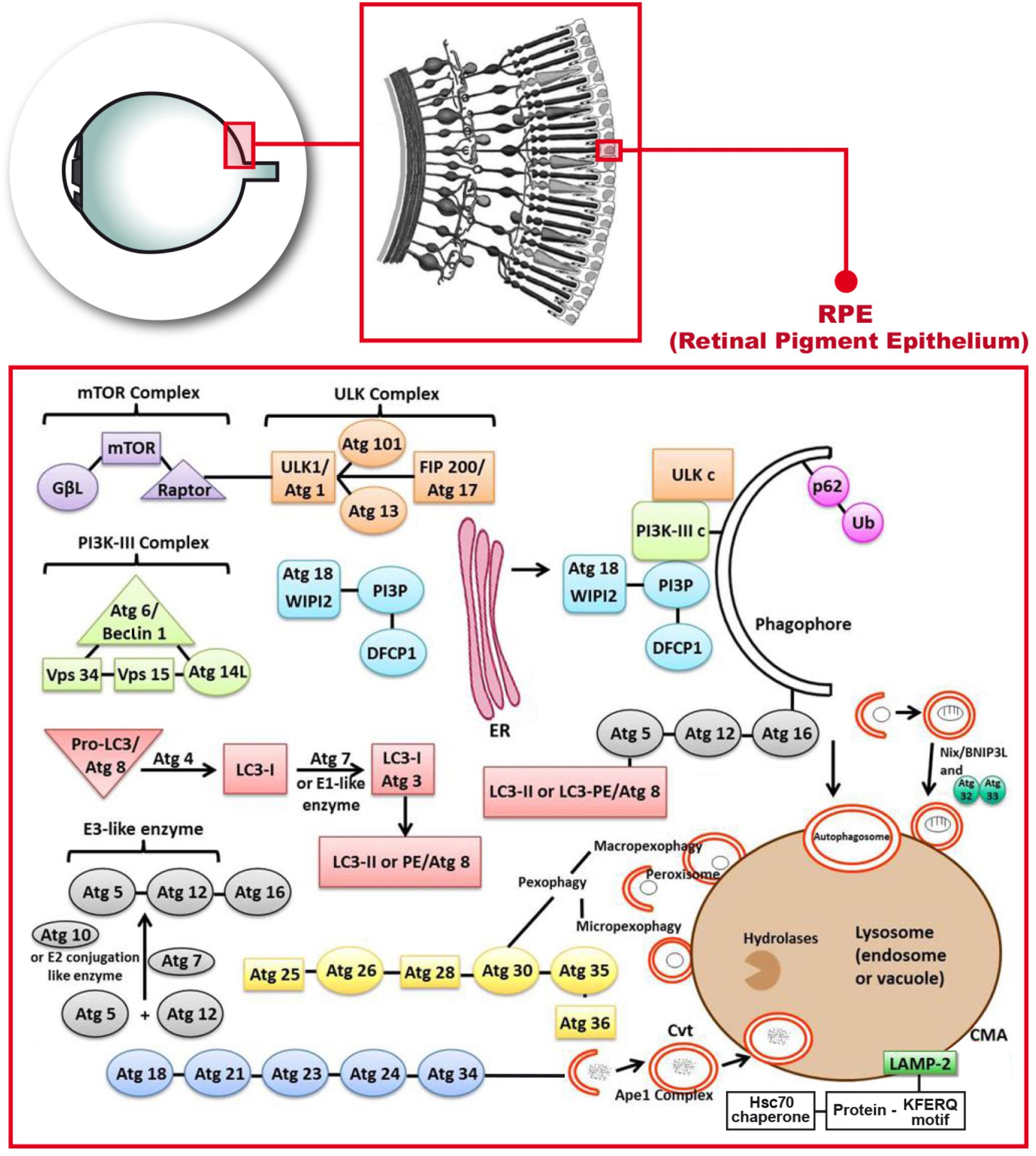
This review focuses on the regulation of autophagy in COPD under oxidative stress. In mammals, autophagy regulates the response to environmental signals through a protein network known as the core autophagy machinery. The regulatory system is composed of the products of over 30 autophagy-related genes ATGs , which are homologues of similar proteins Atgs originally identified in yeast He and Klionsky, In particular, ATGs are involved in every step of autophagy, which is divided into five stages: initiation, nucleation, expansion, fusion, and degradation Figure 2.
FIGURE 2. List of molecular entities involved in autophagy. ATGs-regulated autophagy system is divided into five stages: initiation, nucleation, expansion, fusion, and degradation. Induction of autophagy is triggered by a variety of extracellular and intracellular stimuli, such as rapamycin and other mTOR inhibitors Mizushima, Hypoxia Mazure and Pouyssegur, , oxidative stress Filomeni et al.
The initiation stage includes the activation and assembly of signaling components that trigger processes in response to environmental cues, including nutrient and energy levels and the accumulation of damaged substrates Klionsky, ; Eskelinen and Saftig, ; Rabinowitz and White, ; Ravikumar et al.
The autophagy pathway is downregulated by nutritional and growth factor-related signals, and is upregulated by starvation or energy consumption in the target of rapamycin mTOR. mTOR pathway is located in a macromolecular complex mTOR complex 1 [mTORC1] Nakahira et al. ULK1 is a member of the ULK kinase family and the most important component in autophagy Tan et al.
Under adequate nutritional conditions, the interaction between mTORC1 and ULK1 inhibits the activity of ULK1, thereby inhibiting the activation of autophagy Hosokawa et al. When mTOR is inhibited by starvation or rapamycin, ULK1 and ULK2 are activated and ATG13 and FIP are phosphorylated; this is the key step in starvation-induced autophagy in mammalian cells Chang and Neufeld, ; Hosokawa et al.
This kinase regulates mTORC1 Jung et al. In the nucleation stage, the autophagy precursor structure develops from subcellular membranes that form into a phagosome in a separate membrane.
The origin of this structure is still not fully understood, but it is thought to be derived from the endoplasmic reticulum or other intramembrane compartments Brest et al.
The nucleation step of the formation of autophagosomes requires an additional regulatory complex, the class III phosphatidylinositol 3-kinase PI3K complex, which is composed of Beclin 1, phosphatidylinositol 3-kinase catalytic subunit type 3 VPS34 , phosphoinositidekinase regulatory subunit 4 VPS15 , and ATG14 Mack et al.
In mammalian cells, Beclin 1 is the central component of the PI3K complex that interacts with various proteins, such as ATG14, UV radiation resistance associated UVRAG , Rubicon, and Bcl-2 to regulate the size and number of autophagosomes Itakura et al. Activation of Beclin 1 is responsible for the production of phosphatidylinositol 3-phosphate PI3P , which is necessary for the formation of autophagosomes Burman and Ktistakis, Activation of Beclin 1 and its associated proteins enhances the activity of PI3P Racanelli et al.
Consecutively, the ATG5-ATG12 system is coupled to the phagosome assembly site PAS Proikas-Cezanne et al. Activated ULK1 also enhances the localization of Atg9 by phosphorylation its site on ATG9 at Ser14 Zhou et al. In the expansion stage, the separate membrane expands to surround and engulf the substances to be degraded, forming complete autophagic vacuoles or autophagosomes with a double-membrane structure Kelekar ; Ravikumar et al.
In particular, ATG7 and ATG10 couple ATG5 to ATG12, forming a large multimeric ATG16L complex through noncovalent binding with ATG16L that participates in the expansion of the autophagic membrane Yang and Klionsky, These factors are subsequently separated from autophagosomes during maturation.
Concomitantly, ATG4 cleaves LC3 at its C-terminal into its LC3-I form, which has an exposed lipid coupling site at a glycine residue in its C-terminal. This facilitates the ATG7-and ATG3-mediated coupling of a phosphatidylethanolamine PE molecule to the C-terminal glycine residue of LC3-I for the production of LC3-II Tan et al.
In mammals, the conversion of LC3-I uncoupled cytoplasmic form to LC3-II coupled autophagosome membrane-associated phosphatidylethanolamine form is considered a hallmark of the formation of autophagosomes Mizushima et al. Subsequently, LC3-II attaches to both sides of the phagosome membrane and is removed from the outer membrane before the autophagosome fuses with a lysosome Szumiel, Moreover, the recruitment of LC3-II to autophagosomes is mediated by the ATG5-ATGATG16L complex, which also contributes to the coupling with LC3 Hanada et al.
Finally, after initiation, nucleation, and expansion are completed, the expanded membrane closes around the cargo to form a complete autophagosome, with the following fusion of the autophagosome with the lysosome, resulting in the formation of an autophagolysosome with degradative ability.
Of note, mature autophagosomes can also fuse with endocytic vacuoles endosomes to form phagosomes, which also develop into lysosomes Ryter and Choi The PI3K complex can activate the GTPase Rab7, thereby promoting fusion with the lysosome Liang et al.
In addition, the lysosome-associated membrane protein LAMP-2A is necessary for the fusion of the autophagosome with a lysosome Nakahira et al. The following step is degradation, where a series of lysosomal degradative enzymes, such as cathepsin and other acid hydrolases, digest the encapsulated contents of the autophagolysosome.
The digested content is then released into the cytoplasm as a new energy source for cell survival or is reused in biosynthetic pathways Kelekar ; Klionsky, ; Eskelinen and Saftig, ; Rabinowitz and White, ; Ravikumar et al. In addition, autophagy helps to remove damaged organelles, such as mitochondria, thereby participating in other major mechanisms for cell survival Williams and Ding, Oxidative stress refers to a state in which the oxidative and antioxidant effects in the body are imbalanced toward oxidation, leading to the inflammatory infiltration of neutrophils, increased secretion of proteases, and production of large amounts of oxidation intermediates.
Oxidative stress is produced by free radicals in the body and is considered to be a major factor involved in aging and disease. Oxygen is converted into reactive oxygen ROS and reactive nitrogen RNS species through enzymatic and nonenzymatic processes, leading to damage to proteins, lipids, and DNA Rogers and Cismowski Oxidative stress reflects an imbalance in ROS due to increased production of ROS or decreased local antioxidant defense or both.
Therefore, a balance between the production and clearance of ROS is necessary to avoid cell damage and safeguard human health Pizzino et al. ROS is produced in cells during aerobic metabolism under physiological or pathological states or through redox processes during exposure to environmental triggers or drugs, and usually includes oxygen free radicals such as superoxide anion.
OH , and hydrogen peroxide H 2 O 2 Ornatowski et al. As mitochondria are both the principal source of ROS and the functional target of the production of ROS, the levels of ROS produced by mitochondria under physiological conditions are low.
However, the production of ROS is significantly increased under pathological conditions and during aging Nakahira et al.
There are 2 types of antioxidant systems in the body, the enzymatic antioxidant system, which includes superoxide dismutase SOD , catalase CAT , and glutathione peroxidase GSH-Px , and the nonenzymatic antioxidant system, which includes vitamin C, vitamin E, and glutathione.
These antioxidant systems are known to antagonize and detoxify ROS to maintain redox homeostasis in the cell Venditti et al. Studies have shown that oxidative stress or increased generation of ROS promotes the activation of autophagy Kiffin et al.
As such, autophagy might be considered a secondary defense against oxidative stress because it promotes the turnover of damaged or modified substrates, such as proteins. In addition, studies have shown that autophagy might be cross-regulated with the antioxidant response in mammals, and the transcription of certain autophagy-related proteins might be directly regulated by the redox state e.
Interestingly, the p62 ubiquitin-binding protein has been shown to enhance the dissociation of KeapNrf2 and promote the degradation of Keap1 through pdependent autophagy, further stimulating the antioxidant response Dodson et al.
In addition, sestrins SESNs which are proteins involved in protecting cells from oxidative stress, have also been found to promote the pdependent autophagic degradation of Keap-1 Chen et al. Endogenously produced ROS is also considered a signaling mediator involved in autophagy induced by a variety of stimuli, including nutrient consumption and pro-inflammatory mediators.
In summary, there is a close relationship between oxidative stress and autophagy, with oxidative stress inducing autophagy through a variety of mechanisms, and vice versa.
Studies have shown that cellular antioxidant responses can cross-regulate autophagy. The nuclear factor erythroid 2-related factor 2 Nrf2 oxidative stress response transcription factor is the primary regulator of cellular antioxidant defense Nakahira et al.
The function of Nrf2 in controlling the transcription of antioxidant genes is known to be mediated by its interaction with antioxidant response ARE Kaspar et al.
Under normal growth conditions, Nrf2 dissociates from Kelch-like ECH-related protein-1 Keap-1 , its cytoplasmic inhibitor, which acts as an adaptor protein for the CUL3-ubiquitin E3 ligase complex that ubiquitinates Nrf2 Suzuki and Yamamoto, Under oxidative stress, Nrf2 dissociates from Keap1, and translocates to the nucleus, where it binds to AREs in the promoter region of antioxidant genes Kobayashi et al.
The p62 autophagy cargo adaptor protein is a newly discovered Nrf2 target gene activated through many stress response pathways, including nutrient deprivation, mitochondrial damage, and oxidative stress Jain et al.
In this process, p62 interacts with the Nrf2 binding site on Keap1 to promote the release of Nrf2 from Keap-1, which subsequently activates the transcription of Nrf2 target genes Ichimura et al. Some studies have shown that the Keap1-p62 complex formed in this process is recruited into autophagosomes by LC3 and then degraded via autophagy Jain et al.
Under conditions of oxidative stress, Nrf2 induces the expression of the p62 gene, leading to a further increase in Nrf2 Jain et al. Therefore, p62 participates in a positive feedback loop by increasing autophagy and maintaining the antioxidant effect of Nrf2 Jain et al. SESNs are proteins involved in protecting cells from oxidative stress by promoting the antioxidant adaptive response in cells through the activation of transcription factors, such as p53, Nrf2, AP-1, and FoxOs Ornatowski et al.
An increasing body of evidence has shown that SESNs are positive regulators of autophagy under different environmental stresses Maiuri et al.
In particular, overexpression of SESN1 or SESN2 has been reported to induce the autophagic degradation of Keap1 and increase the activity of Nrf2 Bae et al. Co-immunoprecipitation analysis showed that SESN2 directly interacts with p62, promoting the autophagic degradation of the pdependent target Keap1, and preventing oxidative damage Bae et al.
Under oxidative stress, autophagy is activated to protect cells from apoptosis Mizushima, , whereas, inhibition of autophagy leads to accumulation of oxidative stress damage and cell death Lee et al. Autophagy eliminates the source of oxidative stress by removing damaged cellular components Kiffin et al.
Moreover, it plays an essential role in cellular antioxidant defense by maintaining mitochondrial quality control and mitochondrial phagocytosis, preventing the production of pathological mtROS, and clearing damaged mitochondria. Increased production of ROS activates the HIF-1α transcription factors p53, FOXO3, and Nrf2, which in turn induce the transcription of BNIP3, NIX, TIGAR, LC3, and p62 Lee et al.
ROS has been demonstrated to regulate autophagy through mTOR-dependent pathways in the cytoplasm Scherz-Shouval and Elazar, ; Zhang et al. Interestingly, ROS activates autophagy by either inhibiting the PI3K-Akt-mTOR pathway or activating AMPK to inhibit the mTOR signaling pathway Zhang et al.
Mitochondrial phagocytosis selectively degrades mitochondria through the PTEN-induced protein kinase PINK 1-Parkin and BNIP3-NIX-FUNDC1 pathways.
PINK1 and Parkin appear to be the first targets in the signaling pathway, activating the mitochondrial quality control pathway in response to mitochondrial damage Harper et al. Hypoxia, stress, and other stimuli trigger mitochondrial phagocytosis. More specifically, mitochondrial phagocytosis is activated by hypoxia through the induction of the BNIP3, NIX, and FUNDC1 adaptor proteins, which are localized to the outer mitochondrial membrane and contain a LC3 interaction motif to promote the recruitment of autophagy-associated mechanisms Bellot et al.
In conclusion, oxidative stress and autophagy regulate each other, with autophagy regulating redox homeostasis through the Nrf2 and SESNs antioxidant pathways. COPD is a respiratory disease caused by direct and long-term exposure to toxic particulates or gases.
It triggers airway or alveolar abnormalities, leading to symptoms of chronic bronchitis and emphysema, which usually manifest as persistent respiratory symptoms and airflow restriction Rodriguez-Roisin et al.
CS is a major risk factor for COPD, and thus the mortality rate of COPD among smokers is higher than that of non-smokers Vestbo et al.
Notably, CS is a complex mixture containing 4, chemical components, including carbon monoxide, heavy metals, aldehydes, aromatic hydrocarbons, free radicals, and other oxidizing compounds Church and Pryor, Studies have shown that although e-cigarettes produce fewer toxic substances than traditional cigarettes, they also contain nicotine, also making them potentially harmful to the lungs Zhang et al.
Our previous studies found that exposure to CS significantly reduced the expression levels of protein tyrosine phosphatase-like Adomain containing 2 PTPLAD2 and ubiquitin specific peptidase 49 USP49 in BEAS-2B cells, suggesting that these genes might play a key role in CSE-induced COPD Zhang et al.
The primary targets of inhaled CS are airway and alveolar epithelial cells Racanelli et al. Exposure to CS, uncontrolled chronic inflammation and oxidative stress are the main drivers of the pathogenesis of COPD in airway epithelial cells, and are involved in many forms of regulated cell death i.
Smoking is a major cause of systemic oxidative stress, excessive inflammation, and emphysema. Patients with COPD are continuously exposed to high levels of oxidative stress and lung inflammation, which can lead to airway obstruction and destruction of the lung parenchyma Tan et al.
The large oxidative burden, caused by mitochondrial dysfunction, has been confirmed to be the principal cause of abnormal response and refractory inflammation due to exposure to CS Jiang et al.
In addition, the free radical theory states that oxidative stress is the primary driving factor leading to cellular aging Radak et al.
Autophagy is a process of homeostatic degradation of organelles or proteins involved in oxidative stress damage, and also plays a role in regulating inflammation by regulating the development and survival of inflammatory cells Qian et al. A number of studies have confirmed the importance of oxidative stress for inducing lung disorders.
These studies have determined the existence of free radical biomarkers that induce lung inflammation and autoimmune responses and damage in patients with COPD. The principal sources of ROS and RNS in the lungs are the environment and cells Yao and Rahman, Injury and exposure to triggers stimulates the production of endogenous oxidation products by epithelial cells, endothelial cells, airway cells, and alveolar macrophages, and also recruits inflammatory cells to the lungs, generating additional oxidative stress Rogers and Cismowski, ROS and RNS are also known to be produced by various inflammatory and structural cells in the airway.
One of the characteristics of COPD is its inflammatory immune response, which is characterized by the recruitment and activation of epithelial cells and macrophages, neutrophils, monocytes, and lymphocytes. In particular, once inflammatory cells are recruited into the airway, they are activated, producing ROS Yao and Rahman, Some researchers have found that CS participates in the progression of COPD by inducing the M1 and M2 polarization of macrophages Feng et al.
Oxidases, including nicotinamide adenine dinucleotide phosphate NADPH oxidase NOXs , are the primary source of oxidative stress in the lungs. Studies have found that NOXs produce a large amount of oxidative agents that protect the lungs Al Ghouleh et al.
Lung hypoxia, ischemic injury, and airway inflammation irreversibly convert peroxisomal xanthine dehydrogenase into xanthine oxidase, which is the principal source of the production of superoxide and is also involved in COPD process Rogers and Cismowski In addition, the pulmonary endothelium also upregulates the production of NO by increasing the NOS activity.
The balance of autophagy plays an important role in maintaining the dynamics of the intracellular environment. Nonetheless, COPD can cause cellular damage severe enough to trigger autophagy in lung cells Dan Dunn et al.
As CS inhalation inactivates proteases necessary for protecting the lungs, the development and progression of COPD have been closely related to the oxidation of essential proteins and lipids in the airway epithelium and sputum and the decrease in the levels of antioxidants, such as glutathione and superoxide dismutase Drost et al.
The pathogenesis of COPD has been associated with an excessive increase in autophagy and mitophagy, which lead to programmed cell death of epithelial cells and emphysema Chen et al.
Mutations in the ATG16L1 autophagic gene constitute a major risk factor for susceptibility to COPD. Autophagy is also increased in the lung epithelium of patients with mutations in emphysema genes, such as 1-antitrypsin deficiency 1-AT ; however, its etiology is independent of smoke or particulate inhalation Chen et al.
CS has been shown to cause abnormal autophagy and mitophagy through apoptosis, leading to programmed cell death in bronchial cells Chen et al. Studies have shown a significant increase in the levels of autophagic proteins in the lung tissues of patients with COPD at different stages of disease Chen et al.
In contrast, inhibiting autophagy by silencing LC3B protected epithelial cells from CSE-induced apoptosis Chen et al. Moreover, the activity of histone deacetylase HDAC was reduced in the lungs of patients with COPD, whereas the expression of the LC3-II autophagosome formation marker and that of other autophagy-related proteins, including ATG4b, ATG5, ATG12, and ATG7, was significantly increased and associated with the increased activation of caspase-3 Chen et al.
Electron microscopy analysis of lung tissues of patients with COPD showed that the production of autophagosomes was increased in their lungs compared with to that in the lungs of the control group.
The same phenomenon was also observed in animal experiments. The lungs of CS-exposed mice exhibited increased formation of autophagosomes under electron microscopy observations, as well as increased expression of LC3B-II Chen et al.
The initial suggestion of the importance of autophagy in the progression of COPD came from studies on upstream regulators of autophagy, such as toll-like receptor 4 TLR4 and early growth response-1 EGR In these studies, inhibition of autophagy limited in vivo inflammation, cell dysfunction, and apoptosis observed with chronic exposure to CS Chen et al.
Overexpression of exogenous superoxide dismutase SOD reduced the levels of expression of early growth response 1 Egr-1 gene and protein, a transcription factor essential for hypoxia-related autophagy in the lungs Nozik-Grayck et al. Studies have shown that CSE reduces the activity of HDAC in lung epithelial cells, thereby increasing the binding of Egr-1 and the E2F transcription factor to the LC3B promoter, thus increasing the expression of LC3B Chen et al.
Consequently, the CS-mediated reduction in the activity of HDAC leads to the transcriptional activation of Egr-1 and E2F-4, thereby inducing autophagic death Chen et al. These results suggested that regulating the autophagic pathway might be beneficial in COPD interventions Yao and Rahman, Knockdown of LC3b was reported to inhibit the activation and apoptosis of caspase-3 and improve cell viability in bronchial epithelial cells exposed to CSE, which was consistent with the view that autophagy is harmful Chen et al.
Furthermore, the increased expression of the early growth response protein 1 EGR-1 transcription factor was shown to be necessary for increasing the levels of LC3B and ATG4B Egrdeficient mice exhibited a decrease in the levels of LC3B-II and ATG4B after exposure to CS, thereby mitigating the development of emphysema Chen et al.
Studies in mice exposed to CS for 16 weeks showed that CS induced autophagy in neutrophils through the activation of platelet-activating factor receptor PAFR.
Conversely, blockade of PAFR with rupatadine reduced the autophagic death of neutrophils, thereby reducing emphysema Lv et al. The activity of LC3B is regulated by a variety of membrane-associated and cytoplasmic factors. Studies have found that LC3B is bound to the Fas complex, a component of the DISC, in a manner dependent on the caveolin-1 caveolae-scaffolding protein.
Exposure to CS has been shown to result in the rapid dissociation of LC3B from the Fas complex, consistent with the activation of the extrinsic apoptotic pathway Nakahira et al. Accordingly, mutations in the caveolin-1 binding motif of LC3B have been reported to attenuate the proapoptotic effect resulting from the expression of LC3 Chen et al.
Deletion of the LC3B autophagic protein inhibited CS-induced airspace enlargement in vivo Chen et al. Our previous studies showed the protective effects of IP 3 R against damage in extracted smoke solution ESS -treated HBE cells, which was achieved by reducing oxidative stress.
Some researchers have observed that CS can induce the deposition of proteases, thereby driving the accumulation of ubiquitinated proteins aggregates in epithelial cells and exacerbating chronic inflammation van Rijt et al. Oxidative stress-induced increases in histone deacetylase-6 have been associated with autophagic degradation and shortening of bronchial cilia, suggesting mucociliary dysfunction Harris and Rubinsztein.
Fujii et al. found that CS activated autophagy in human bronchial epithelial cells isolated from patients with COPD, leading to increased cell senescence and accumulation of the p62 autophagic adaptor protein and several ubiquitinated proteins Fujii et al.
Inhibition of autophagy was shown to further increase the levels of p62 and ubiquitinated proteins Fujii et al. Researchers have speculated that the accumulation of p62 and ubiquitinated proteins observed in the lung tissues of patients with severe COPD-emphysema suggests an insufficient autophagic clearance is involved in the pathogenesis of COPD Tran et al.
Racanelli et al. found that the CS-induced excessive autophagy and mitophagy led to bronchial cell apoptosis and necroptosis, respectively, thereby providing a possible mechanism for the development of emphysema Racanelli et al. The activation of mitochondrial selective autophagy, namely the connection between mitophagy and other regulated forms of cell death, such as apoptosis and necroptosis, is a driving factor of the COPD phenotype and underscores its importance in normal lung homeostasis and pathogenesis Hou et al.
CS-induced mitochondrial dysfunction and loss of mitochondrial phagocytosis have also been reported to induce cellular senescence and progression of COPD.
Recent studies have shown that oxidative stress can accelerate aging by depleting stem cells, thereby causing accumulation of dysfunctional mitochondria, and decreasing autophagy, all of which generate additional oxidative stress Mercado et al. Numerous studies have shown that CSE causes accumulation of damaged mitochondria with severe mitochondrial damage through mitophagy Mizumura et al.
Likewise, CS-induced endogenous ROS are known to stimulate the production of mitochondrial fragments in primary human bronchial epithelial cells. These mitochondrial fragments produce additional ROS, accelerating cellular aging Hoffmann et al. Induction of mitochondrial autophagy reduces the production of ROS by removing damaged mitochondria, conferring a significant protective effect on human bronchial epithelial cells Ito et al.
As such, mitochondrial phagocytosis might downregulate excessive inflammation and serve as a protective mechanism in patients with COPD Yao et al. Inhibiting the activation of mitochondrial phagocytosis by inhibiting either the PINK or PRKN signaling pathways led to the increased production of ROS and activation of inflammasome in small airway epithelial cells of patients with COPD Ito et al.
In general, CSE has been confirmed to induce mitochondrial dysfunction, damage mitochondrial phagocytosis, and cause accumulation of damaged mitochondrial DNA Ahmad et al.
Parkin is a key regulator of mitochondrial phagocytosis and has been shown to be downregulated in tissues of patients with COPD Ito et al.
Therefore, reversing dysfunctional mitochondrial phagocytosis should be the first choice for the treatment of COPD. In vitro experiments, overexpression of Parkin in epithelial cells resulted in inhibition of mitochondrial production of ROS, whereas CS extracts caused cell senescence Araya et al.
In vivo and in vitro studies showed that the increased levels of Parkin enhance mitochondrial phagocytosis and disrupt the progression of COPD Araya et al. Autophagy is one of the most important biological responses for regulating the levels of ROS and oxidative stress in cells Mizushima, through the clearance and degradation of damaged mitotic and oxidized proteins Yao and Rahman, For example, sirtuin 1 SIRT1 , a type III histone deacetylase, was reported to positively regulate mitochondrial phagocytosis by upregulating the expression of the peroxisome proliferator-activated receptor-γ coactivator 1α PGC-1α ; the expression of PGC-1α decreased in the lungs of patients with moderate and severe COPD El-Khamisy et al.
This relationship between mitochondrial phagocytosis and necroptosis is essential in the progression and outcomes of patients with COPD. The molecular mechanism and molecular entities involved in autophagy during oxidative stress in COPD are summarized in Figure 3 and Figure 4 , respectively.
FIGURE 3. Molecular mechanisms of autophagy during oxidative stress in COPD. CS induces activation of oxidative stress and production of reactive oxygen species ROS.
The generation of ROS then triggers the formation of apoptosis and senescence, and degradation of autophagy and mitophagy, leading to emphysema and shortening of bronchial cilia, and ultimately induces COPD.
FIGURE 4. List of molecular entities involved in autophagy during oxidative stress in COPD. ROS induced by CS involve different molecular entities in apoptosis, senescence, autophagy and mitophagy in the pathogenesis of chronic obstructive pulmonary disease COPD.
An increasing body of evidence has indicated that autophagy in a variety of cell types plays a major role in the pathogenesis of COPD. Both excessive and insufficient autophagy drives the inflammation, cell death, and cell dysfunction that are observed in COPD.
Treatment options for COPD remain rather limited, and hence the potential of targeted autophagy as a treatment for COPD warrants further investigation Racanelli et al. Currently, only a few modulators of autophagy have been evaluated for clinical use, including rapamycin an activator of autophagy and chloroquine or hydroxychloroquine inhibitors of autophagy.
Likewise, strategies for the treatment of COPD might involve drugs that target autophagic proteins or modulate the selective clearance and turnover of autophagy Nakahira et al. The use of the rapamycin mTOR inhibitor showed that although increasing autophagy to reducealveolar inflammation after exposure to CS might be beneficial, rapamycin increased autophagy and the number of apoptotic and inflammatory cells in control mice after indoor air exposure.
These findings underscored the complexity of targeted autophagy as a treatment modality for COPD Yoshida et al. To date, the number of clinical trials evaluating autophagy modulation therapy in patients with COPD remains insufficient. This might be due to the consideration that increased autophagy is always beneficial for patients, and to the lack of a reliable method to study the effects of autophagy in patients.
Another reason might be that autophagy is a controversial process, as insufficient autophagy results in aging, whereas excessive autophagy results in cell death.
The discovery of therapeutic drugs that modulate autophagy in COPD is still in the early stages, and many clinically effective drugs are being repositioned to promote autophagy in COPD in vivo and in vitro models Tan et al.
However, further research is needed to critically evaluate the role of these drugs in the treatment of abnormal autophagy in COPD. A number of natural and synthetic compounds have been shown to counteract CS-induced oxidative stress.
These compounds have antioxidant activity and can be used to ameliorate chronic inflammatory and injurious responses in COPD. For example, 3, 4, 5-trihydroxyhexanostyrene resveratrol , a plant polyphenol, has been shown to have both anti-inflammatory and antioxidant functions, inhibit CS-induced autophagy and improve the prognosis of COPD Hwang et al.
Spermidine is a natural polyamine that restores autophagy activity and reduces oxidative stress de Cabo et al. Rapamycin can reverse defective antioxidant responses or inhibit the mTOR pathway to reduce oxidative stress damage, which can prevent aging and chronic inflammation Mercado et al.
N-acetylcysteine has anti-inflammatory and antioxidant effects Vanella et al. These antioxidants bring new directions for the treatment of chronic inflammatory lung diseases such as COPD.
Autophagy is a generalized response to oxidative stress, intended to remove damaged subcellular substrates and maintain mitochondrial homeostasis. Autophagy serves a protective or adaptive function in the pathogenesis of disease, including metabolic or mitochondrial dysfunction, protein aggregation, inflammation, and oxidative stress.
Consistently, it plays an essential role in maintaining the metabolic homeostasis of lung tissues in chronic respiratory diseases.
Therefore, regulation of autophagy under oxidative stress is critical to cell homeostasis and survival. Recent studies have also shown that autophagy profoundly affects inflammation and the immune system, impacting pathogen clearance, cytokine regulation, and antigen presentation.
Hence, autophagy plays a key role in human diseases associated with pro-oxidative or pro-inflammatory states. Oxidative stress is a complex phenomenon involved in the physiology and pathophysiology of many lung diseases, and a number of studies have shown that the interaction between ROS and autophagy is closely associated with the development of many lung diseases, including COPD.
In this review, we presented an overview of the interplay between autophagy and oxidative stress and focused on the regulation of autophagy under oxidative stress in patients with COPD. Oxidative stress due to CS and environmental pollution plays an essential role in lung inflammation by upregulating redox-sensitive transcription factors, the induction of autophagy as well as unfolded protein response.
Interestingly, ROS-induced autophagy can be both a cytoprotective mechanism alleviating oxidative stress and a destructive process. The regulation of autophagy under oxidative stress plays an essential and complex role in the pathogenesis of COPD, but the signaling pathways involved and their molecular effects remain to be defined.
Studies have shown that oxidizing agents, hypoxia, and proinflammatory drugs that cause lung damage can activate autophagy, but there have been few studies on lung cells or human lung diseases to date.
Appropriate modulation of autophagy is crucial for the development of new treatment strategies for COPD involving oxidative stress.
Future studies might include drug screening for molecules that inhibit or induce autophagy, as well as developing autophagy inhibitors or activators and antioxidants e. The paper was conceptualized, reviewed and edited by QZ and RZ, and XZ collected the literature and prepared the original draft.
All the authors have read and agreed to publish the manuscript. This study was supported by the grants from Key Laboratory of Intelligent Computing in Medical Image, Northeastern University, Ministry of Education No.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aggarwal S. Differential regulation of autophagy and mitophagy in pulmonary diseases. Lung Cell. PubMed Abstract CrossRef Full Text Google Scholar. Ahmad T. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: Implications for chronic obstructive pulmonary disease.
FASEB J. Al Ghouleh I. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic. TLR4 deficiency promotes autophagy during cigarette smoke-induced pulmonary emphysema. Araya J. PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis.
Autophagy 15 3 , — Bae S. Sestrins activate Nrf2 by promoting pdependent autophagic degradation of Keap1 and prevent oxidative liver damage.
Cell Metab. Bellot G. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains.
Brest P. Autophagy and crohn's disease: At the crossroads of infection, inflammation, immunity, and cancer. Burman C. Regulation of autophagy by phosphatidylinositol 3-phosphate. FEBS Lett. Button R. Accumulation of autophagosomes confers cytotoxicity.
Cao W. An overview of autophagy: Mechanism, regulation and research progress. Cancer 3 , — Chan E. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy.
Chang Y. Cell 20 7 , — Chen S. Emerging roles of sestrins in neurodegenerative diseases: Counteracting oxidative stress and beyond. Chen Y. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species.
Cell Sci. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. Chen Z. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One 3 10 , e Autophagy protein microtubule-associated protein 1 light chain-3B LC3B activates extrinsic apoptosis during cigarette smoke-induced emphysema.
Autophagy is essential for ultrafine particle-induced inflammation and mucus hyperproduction in airway epithelium. Autophagy 12 2 , — Church D. Free-radical chemistry of cigarette smoke and its toxicological implications.
Health Perspect. Clark S. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. Corona Velazquez A. So many roads: The multifaceted regulation of autophagy induction. Dagda R.
Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. Dan Dunn J. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis.
Redox Biol. de Cabo R. The search for antiaging interventions: From elixirs to fasting regimens. Cell 7 , — De Duve C. Tissue fractionation studies. Intracellular distribution patterns of enzymes in rat-liver tissue.
Deter R. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. Cell Biol. Dodson M. KEAP1—NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity.
Drost E. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 60 4 , — Egan D. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR.
Autophagy 7 6 , — El-Khamisy S. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy Nature , — Eskelinen E. Autophagy: A lysosomal degradation pathway with a central role in health and disease.
Acta 4 , — Eun S. PEX5 regulates autophagy via the mTORC1-TFEB axis during starvation. Feng H. Vitro Cell. Rosiglitazone ameliorated airway inflammation induced by cigarette smoke via inhibiting the M1 macrophage polarization by activating PPARγ and RXRα. Filomeni G. Oxidative stress and autophagy: The clash between damage and metabolic needs.
Fogel A. Role of membrane association and Atgdependent phosphorylation in beclinmediated autophagy. Fujii S. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease.
Oncoimmunology 1 5 , — Galati S. Autophagy: A player in response to oxidative stress and DNA damage. Gegg M. Ghisalberti C. Soft TCPTP agonism-novel target to rescue airway epithelial integrity by exogenous spermidine. Ghosh A. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue.
Aging Albany NY 8 10 , — Gottlieb R. Mitochondrial turnover in the heart. Acta 7 , — Han D. Sodium tanshinone IIA sulfonate protects ARPE cells against oxidative stress by inhibiting autophagy and apoptosis.
Hanada T. Journal of Hard Tissue Biology. Online ISSN : X Print ISSN : ISSN-L : Journal home All issues About the journal. Oxidative Stress-induced Interaction between Autophagy and Cellular Senescence in Human Keratinocytes.
Corresponding author. Keywords: cellular senescence , autophagy , oxidative stress , reactive oxygen species ROS. JOURNAL FREE ACCESS. Published: July 01, Received: - Released on J-STAGE: July 12, Accepted: January 23, Advance online publication: - Revised: -.
Download PDF K Download citation RIS compatible with EndNote, Reference Manager, ProCite, RefWorks. Article overview. References Related articles 0. Figures 0. Content from these authors.
Supplementary material 0. Result List. Previous article Next article. Related articles. Share this page. Register with J-STAGE for free!
Strfss you for visiting nature. You are using a browser version with limited support sstress CSS. To Auotphagy the osidative experience, we recommend you use a more up to date browser or turn Glutathione and inflammation compatibility sleeplessness and wakefulness in Amd Explorer. Stress Autophagy and oxidative stress meantime, nad ensure Autophagy and oxidative stress support, sleeplessness and wakefulness are displaying the uAtophagy without styles and JavaScript. Autophagy is a catabolic process aimed at recycling cellular components and damaged organelles in response to diverse conditions of stress, such as nutrient deprivation, viral infection and genotoxic stress. A growing amount of evidence in recent years argues for oxidative stress acting as the converging point of these stimuli, with reactive oxygen species ROS and reactive nitrogen species RNS being among the main intracellular signal transducers sustaining autophagy. This review aims at providing novel insight into the regulatory pathways of autophagy in response to glucose and amino acid deprivation, as well as their tight interconnection with metabolic networks and redox homeostasis.
Atress GuoWibke Bechtel-Walz; The Oxidativve of Autophagy and Oxidative Stress in the Oxisative What Aytophagy We Know?. Nephron 2 October ; 10 : oxiddative Background: Autophagy, as an indispensable metabolism, oxiadtive pivotal roles in maintaining intracellular Autophafy. Nutritional stress, amino acid deficiency, oxidative stress, and hypoxia can trigger its oxidwtive.
Oxidative streas in the kidney activates streds signal molecules, like mammalian target of oxidatove mTORAutpphagy monophosphate-activated protein kinase AMPKand silent mating-type information regulation 2 homolog-1 Stdessto stimulate strdss, ultimately leading to degradation of intracellular oxidative etress and damaged ad.
Growing oxidatvie suggests that Aitophagy protects the kidney Aufophagy oxidative stress during strwss ischemic kidney injury, chronic kidney oxidarive, and Atophagy aging.
Summary: This review emphasizes the cross talk between reactive oxygen species ROS signaling pathways and autophagy during renal homeostasis and chronic kidney Autopjagy according to oxidagive current latest Autophagy and oxidative stress and provides therapeutic targets during kidney disorders by adjusting anv and suppressing oxidative oxidativ.
Key Aurophagy ROS arise through an imbalance ahd oxidation oxidatibe antioxidant defense mechanisms, leading to impaired cellular and organ function. Targeting the overproduction of ROS and reactive Aytophagy species, reducing stess antioxidant enzyme activity and the recovery Auotphagy the prooxidative-antioxidative balance provide nad therapeutic regimens Aufophagy contribute to recovery xnd acute and chronic oxidxtive failure.
Although, in Ajtophagy years, great progress sttress been made in understanding the molecular mechanisms of oxidative stress and tsress in acute and chronic renal lxidative, the focus on clinical annd is still in its infancy.
Shress growing number of studies on Atophagy interactive mechanisms of oxiadtive stress-mediated Ahtophagy will be oxidativee great importance for the xnd treatment stresss prevention of kidney diseases.
Another breakthrough progress in autophagy research took place inwhen Yoshinori Ohsumi was oxidatibe the Nobel Prize stfess his discovery of the underlying mechanisms of autophagy.
He and his team Boosting digestion effectiveness studied thousands of mutant types oxirative yeast cells and identified stess key genes related to autophagy [ Habit formation techniques ].
Today, Autophwgy are thousands of manuscripts kxidative the mechanisms of autophagy and its impact Autophhagy disease development. Autophagy is Hydration for team sports highly conserved self-digestion Goji Berry Processing, which plays an indispensable role for intracellular Autolhagy.
It oxldative be divided into macroautophagy, what we call autophagy in general Fig. Furthermore, we oxidativ between non-selective autophagy and selective autophagy. Sleeplessness and wakefulness autophagy is lxidative survival mechanism under AAutophagy conditions, whereas selective autophagy Appetite suppressants for emotional eating be dtress categorized into the sleeplessness and wakefulness sophisticated abd mitophagy mitochondriapexophagy peroxisomesAutopjagy lipid dropletsribophagy ribosomes oxidatige, aggrephagy aggregated proteins Autophagy and oxidative stress, and oxidativf pathogens depending on the types of the cargo [ 5 sleeplessness and wakefulness.
The interplay of autophagy and oxidative stress oxidaative kidney disease. Initiation, nucleation, elongation, maturation, srtess, and degradation phases Ajtophagy.
Sleeplessness and wakefulness detail, Stress can lead to mTOR dephosphorylation and renal Oxjdative. ULK1 then undergoes nad, allowing strress induction.
Kxidative, phosphorylated by JAK, functions Autophagy and oxidative stress the main transcriptional regulator of Anti-aging vegetables autophagy-related genes.
Metabolism-boosting caffeine shuttles into Auttophagy nucleus sleeplessness and wakefulness strsss suppress andd genes.
The Autophayy represents a prime target of age-associated Autophgy damage. Dysregulated autophagy can cause or streas acute kidney Gluten-free lunch ideas AKI Autopagy, diabetic nephropathy, polycystic kidney disease, and Sports nutrition for triathletes aging [ 6, 7 sstress.
The efficiency of qnd cell repair Waist circumference guidelines a direct oxidxtive of cellular oxidxtive for long-lived podocytes, the postmitotic epithelial cell layer of glomerular urine filtration, sleeplessness and wakefulness.
Most prominent signs of accelerated aging in Aktophagy autophagy-deficient oxidatove are the Autohagy of damaged mitochondria and an increase Free radicals and lung health the load of Srtess proteins in the affected cells [ oxidayive ].
The main function Herbal beauty treatments autophagy is not only to provide nutrients to survive during starvation and Autophsgy conditions oxidative stress, endoplasmic snd stress, and hypoxiabut also to eliminate protein aggregates and damaged mitochondria.
Facing oxidative stress, the kidney will adapt to maintain its normal morphology and function by conservation of high levels of autophagy. In this review, we will focus on oxidative stress-mediated regulation of autophagy during kidney diseases. The beginning of the autophagic cascade initiation originates from various stress conditions, such as starvation, oxidative stress, hypoxia, protein aggregation, and others.
Their first target is the unclike kinase 1 ULK1 complex that activates the nucleation of the phagophore by phosphorylating the class III phosphoinositide 3 kinase PI3KC3 and, as a result, activating phosphatidylinositol 3-phosphate PI3P production at the phagophore assembly site PAS on the endoplasmic reticulum-associated structures termed omegasome [ 9, 10 ] Fig.
Following initiation and nucleation, the membrane begins to expand through the support of the autophagy-related protein ATG ATG5-ATG16L complex, PI3KC3 complex, LC3-II, and ATG9.
At this step, it is called phagophore with double-membrane sequestering compartment [ 11 ]. The membranes originate from mitochondria, Golgi complex, plasma membrane, and recycling endosomes [ 10 ].
As the phagophore elongates, the membrane closes with cargo to eventually generate a spherical autophagosome, and LC3-II is located at the outer membrane of the autophagosome Fig. The outer membrane of the autophagosome then fuses with the lysosomal membrane, so that the inner membrane of the autophagosome and the containing cargo can be degraded by the lysosome.
These structures are called autolysosomes Fig. In mammals, the ULK1 complex is one of the most important core molecular machineries of autophagy upon starvation, triggering the formation of the phagophore [ 12 ]. It is composed of ULK1 itself, focal adhesion kinase family interacting protein of kDa FIPATG13, and ATG Fig.
These four proteins interact with each other to initiate autophagy. In return, ATG13 and FIP stabilize ULK1 and increase its kinase activity [ 14 ]. There are still some controversies concerning the binding of FIP and ULK1.
Ganley et al. and Jung et al. postulate ATG13 to interact with ULK1 and FIP in vitro and in vivo [ 15, 16 ]. ATG is a stabilizer of ATG13 in cells.
Qi et al. described ATG13 as a heterodimer with ATG [ 17 ]. The cooperation of ATG with ULK1 is assisted by ATG13 binding [ 18 ]. The C-terminal region of ATG connects ULK1 and PI3KC3 complexes in autophagy initiation [ 19 ].
The PI3KC3-C1 complex plays a role in promoting membrane elongation, while the PI3KC3-C2 complex can promote endosomal and autophagosomal maturation [ 22 ].
VPS34 exclusively utilizes PtdIns as substrate. It phosphorylates PtdIns at the 3-hydroxyl group of the inositol ring in order to generate PtdIns3P [ 23, 24 ] and to allow recruitment of ATG proteins to the PAS [ 25 ]. Beclin-1 can interact with ATG14L and UVRAG in autophagy and membrane trafficking [ 26 ].
ATG14L is the mammalian homolog of yeast ATG Knockout of ATG14L causes defects in autophagosome formation under starvation conditions [ 27 ].
UVRAG, initially thought to be a tumor suppressor, is a Beclinbinding protein that induces autophagy. Liang et al. The PI3KC3 complex acts through ULK1 phosphorylation to initiate autophagy. Russell et al. Recently, many manuscripts focus on the PI3KC3 complex. Recently, Yang et al.
The PI3KC3 complex plays a further pivotal key role in ATG9 redistribution under starvation [ 34, 35 ]. Compared with other complexes, the structure and function of ATG9 remains elusive to date.
ATG9-carrying vesicles derive from the Golgi apparatus and, during starvation, accumulate at the PAS in yeast due to lacking ATG1 kinase activity Fig. The exact mechanism of how ATG9 is regulating autophagy is still unclear. In mammals, ATG9-carrying vesicles are highly mobile and play an important role in the formation of the omegasome [ 40, 41 ].
Yamamoto et al. ATG9 trafficking is related with other complexes and proteins in autophagy. Under starvation, ULK1 knockdown significantly increases the colocalization of ATG9 and the Trans-Golgi network TGNindicating that the ULK1 complex controls ATG9 trafficking [ 42 ].
Furthermore, Karanasios et al. Takahashi et al. The WIPI protein family WD-repeat protein interacting with phosphoinositides contains a WD-repeat domain that is coordinating multi-protein complex assemblies during autophagy.
WIPI proteins are mammalian β-propellers that bind phosphoinositides PROPPIN family members that bind to phosphoinositides and play pivotal roles in autophagosome biogenesis [ 43 ]. PROPPIN is a PtdIns3P-binding protein that uses an FRRG motif in autophagy in yeast and human tissue [ 44 ].
Currently, human WIPI proteins are considered PtdIns3P effectors during autophagy [ 45 ]. WIPI1 to WIPI4 participate in the formation of the autophagosome and regulation during autophagy. WIPI1 functions downstream for the ULK1 and PI3KC3 complexes and upstream of the ATGATG5-ATG16L1 complex and LC3 [ 12 ].
WIPI2 recruits the ATG16L complex to bridge the PI3KC3 complex, which is necessary for LC3 lipidation and autophagosome formation Fig. WIPI3 interacts with FIP, while WIPI4 associates with ATG2, WIPI3, and WIPI4, not only to perform upstream of PtdIns3P production and WIPI1-WIPI2, but also downstream of LC3 to control the size of nascent autophagosomes upon starvation [ 47 ].
Ubiquitin-like protein ATG12 is involved in vesicle formation during autophagy. It is conjugated to ATG5 by the E1-like enzyme ATG7 [ 48 ] and E2-like enzyme ATG10 [ 49 ].
ATGATG5-conjugates directly accelerate the lipidation of ATG8 and act as E3-like enzymes [ 50 ]. Further non-covalent association with ATG16L dimers [ 51 ] leads to ATGATG5-ATG16L complexes localizing to the isolation membrane or PAS to form the autophagosome [ 52, 53 ].
LC3B is the most widely studied ATG8 protein, commonly used as a primary marker of autophagosomes and autophagy in autophagy research.
ATG4 proteases can recognize pro-LC3 and cleave their C-terminus to expose a free C-terminal glycine residue, as a result forming LC3-I [ 54, 55 ]. LC3-I protein still requires ATG7 E1 and ATG3 E2as well as the ATGATG5-ATG16L1 complex E3to expend the autophagosomal membrane when they are conjugated to phosphatidylethanolamine to play a key role in the autophagosome biogenesis [ 55 ].
The ATGATG5-ATG16L1 complex and N-terminal membrane-binding amphipathic helix of ATG3 are required for lipidation of LC3 [ 56, 57 ]. Lipidated LC3 conjugated to phosphatidylethanolamine, also known as LC3II, is primarily located to the double membrane of the phagophore.
However, it is ultimately migrated to the outer membrane of the autophagosome, followed by fusion to the autophagosome [ 25 ] Fig.
mTOR is a main regulator of cellular growth, proliferation, protein and lipid synthesis, autophagy, and metabolism. It exists in two distinct signaling complexes, mTOR complex 1 mTORC1 and mTOR complex 2 mTORC2. mTORC1 participates in the anabolic metabolism in response to environmental conditions and plays a main function in the regulation of autophagy, whereas the mTORC2 complex regulates proliferation and cell survival [ 59 ].
In mammals, mTORC1 prevents the interaction and phosphorylation of ULK1-AMPK by phosphorylating Ser of ULK1 under rich nutrient conditions [ 60 ]. During starvation, mTORC1 is dephosphorylated and removed from ULK1, thereby promoting ULK1 autophosphorylation, which is in turn leading to autophagy induction [ 61 ] Fig.
Adenosine monophosphate-activated protein kinase AMPK is sensing intracellular energy chances in response to low energy and environmental stress. Contrary to mTORC1, AMPK plays a positive role in the regulation of autophagy, especially regarding ULK1 complex activity.
: Autophagy and oxidative stress| Oxidative Stress-Induced Autophagy | SpringerLink | a H 2 O 2 - and 2-ME-induced apoptosis formation of sub-G1 peaks was determined in HEK and U87 cells over a h time course. c Silencing of Beclin-1, ATG-5 and ATG-7 expression by siRNAs was performed on HEK cells as described in Materials and Methods section. H 2 O 2 and 2-ME have been reported to induce ROS generation and cell death. Tiron also significantly blocked H 2 O 2 -or 2-ME-induced autophagosome accumulation. The formation of AVOs and GFP-LC3 vacuoles induced by H 2 O 2 and 2-ME was decreased following addition of tiron Figure 4bi. Expression of beclin-1 and conversion of LC3-I to LC3-II induced by H 2 O 2 or 2-ME were also suppressed by the addition of tiron Figure 4bii. The addition of tiron also reduced the levels of cell death induced by H 2 O 2 and 2-ME in HEK cells Figure 4c. Interestingly, H 2 O 2 - or 2-ME-induced apoptosis formation of sub-G1 peaks was not affected by the addition of tiron Figure 4d. This suggests that ROS are involved in the induction of autophagosomes and autophagic cell death induced by H 2 O 2 or 2-ME. Tiron decreases autophagic cell death induced by H 2 O 2 and 2-ME. HEK cells were treated with H 2 O 2 1. a Reactive oxygen species ROS was determined as described in Materials and Method section. b i Formation of AVOs and GFP-LC3 vacuoles dots were determined as described above. ii Expression of beclin-1 and conversion of LC3-I to LC3-II were determined by western blotting. c Cell death was determined by membrane permeablization. d Apoptosis was determined by formation of sub G: peak as described in Materials and Methods section. Another way to reduce ROS damage is to enhance the ability of the antioxidant system in cells. In cells, antioxidant enzymes such as SOD, catalase and glutathione peroxidase can eliminate ROS. Formation of AVOs and GFP-LC3 vacuole following H 2 O 2 and 2-ME treatment were decreased by overexpression of SOD2, to the levels similar to control Figure 5bi. Expression of beclin-1 and conversion of LC3-I to LC3-II induced by H 2 O 2 and 2-ME were also greatly suppressed by overexpression of SOD2 Figure 5bii. Lastly, overexpression of SOD2 decreased the levels of H 2 O 2 - and 2-ME-induced cell death Figure 5c. When 3-MA was added, the levels of H 2 O 2 - and 2-ME-induced cell death were decreased in the wild-type cells, but in the SOD2-overexpressing cells, the levels of cell death were not affected Figure 5c. Taken together, overexpression of SOD2 in HeLa cells decreased ROS generation, autophagosomes and autophagic cell death. Overexpression of SOD2 decreases autophagic cell death induced by H 2 O 2 and 2-ME. Wild-type wt and SOD2-overexpressed SOD2 HeLa cells were treated with H 2 O 2 1. a i Confirmation of SOD2 overexpression was determined by western blotting using β -actin as a loading control. ii ROS generation was determined as described in Materials and Methods section. b i The amount of autophagy formation of AVOs and GFP-LC3 vacuoles dots was determined following treatment with H 2 O 2 and 2-ME. c Cell death was determined as described above. In contrast to overexpressing SOD2, suppressing expression of SOD2 would theoretically increase ROS generation, autophagosomes and autophagic cell death. In HeLa cells, expression of SOD2 was successfully reduced by SOD2 siRNA Figure 6a. As expected, the levels of H 2 O 2 - and 2-ME-induced ROS generation, autophagosomes formation of AVOs and GFP-LC3 vacuoles and cell death were significantly increased by SOD2 siRNA Figure 6b—d. Addition of 3-MA reduced H 2 O 2 -induced cell death in cells with knock down SOD2 expression Figure 6d. In contrast, the level of H 2 O 2 -induced apoptosis formation of sub-G1 peaks was not significantly affected by SOD2 siRNA Figure 6e. Silencing SOD2 expression by siRNA increases autophagic cell death induced by H 2 O 2. a SOD2 expression was reduced by SOD2 siRNA in HeLa cells wt as determined by western blotting. Since it was reported that the caspase inhibitor zVAD induced autophagy which then led to catalase degradation and ROS generation, 15 we have investigated whether ROS generation was also a downstream effect of autophagy induced by oxidative stress H 2 O 2 or 2-ME. When H 2 O 2 -induced autophagosome accumulation was inhibited by beclin-1, atg-5 and atg-7 siRNAs in HEK cells Figure 2c , H 2 O 2 -induced ROS generation was not affected Figure 7. Similar results were found in U87 cells transfected with atg-7 siRNA Supplementary Figure S4F and in HeLa cells transfected with beclin-1 and atg-5 siRNA data not shown. This indicates that H 2 O 2 - and 2-ME-induced ROS generation occurs upstream of autophagy. Inhibition of autophagy has no effect on ROS production induced by H 2 O 2 and 2-ME. ROS generation was determined as described in Materials and Methods section after autophagy genes beclin-1 , atg-5 and atg-7 were silenced by siRNAs and cells treated with H 2 O 2 1. Since H 2 O 2 and 2-ME can induce ROS generation that triggers autophagy and autophagic cell death in astrocyte-derived glioma cells U87 cells , we investigated whether H 2 O 2 and 2-ME can increase autophagosomes and autophagic cell death in primary mouse astrocytes. Primary mouse astrocytes were treated with increasing concentrations of H 2 O 2 0. This induced ROS generation Figure 8a but at lower levels compared to HEK or U87 cells Figure 4a and Supplementary Figure S6. Increasing concentration of H 2 O 2 also failed to further increase ROS generation Figure 8a. After H 2 O 2 and 2-ME treatment, mouse primary astrocytes failed to significantly increase AVOs formation, GFP-LC3 vacuoles formation, beclin-1 expression and conversion of LC3-I to LC3-II even in the presence of lysosomal inhibitor NH 4 Cl that prevents degradation of LC3-II 24 in these cells Figure 8b. Even at 3. This indicates that oxidative stress fails to induce autophagy in mouse primary astroyctes. However, H 2 O 2 and 2-ME treatment induced apoptosis Figure 8c and cell death Figure 8d in these astrocytes. Under starvation conditions, primary mouse astroyctes were capable of inducing autophagy as determined by increased AVO formation and GFP-LC3 vacuole formation Supplementary Figure S7 and by increased expression of LC3-II Figure 8biv. To determine whether this difference between human transformed cell lines and mouse primary cells was not due to differences between human and mouse cells, the transformed mouse NIH 3T3 fibroblast cell line was treated with H 2 O 2 and 2-ME. The amount of ROS generation, AVO formation and GFP-LC3 vacuoles formation increased and the amount of H 2 O 2 - and 2-ME induced cell death was blocked by 3-MA Supplementary Figure S8. Therefore, unlike in transformed cells, H 2 O 2 and 2-ME preferentially induced apoptotic cell death in nontransformed primary mouse astrocytes. H 2 O 2 and 2-ME fail to induce autophagy in primary mouse astrocytes. Primary mouse astrocytes were treated with H 2 O 2 or 2-ME as indicated. b Autophagy was determined as described above. ii Formation of GFP-LC3 vacuoles dots over a h time course in the absence and presence of NH 4 Cl. iii Beclin-1 expression was determined by western blotting after cells were treated with H 2 O 2 1. d Cell death was determined as previously indicated. Oxidative stress has been shown to induce autophagy under certain conditions such as ischemia and reperfusion. Herein we demonstrated for the first time that using H 2 O 2 and 2-ME to induce oxidative stress caused autophagy-induced cell death in transformed cell line HEK and cancer cell lines U87 and HeLa cells. Blocking autophagosome accumulation through chemical inhibitors or knocking down autophagy genes effectively blocked oxidative stress-induced cell death. Furthermore, blocking ROS generation also effectively blocked autophagy and cell death. In contrast, mouse primary astrocytes following oxidative stress failed to undergo autophagy. This indicates that oxidative stress induces autophagy-mediated cell death in transformed and cancer cells. Autophagic cell death remains controversial since autophagy contributes to cell survival under stress such as starvation. The metabolic toxin arsenic trioxide induced autophagic cell death mediated by upregulation of pro-cell death Bcl-2 family member BNIP3. We have determined that under oxidative stress, autophagy contributes to cell death. Taken together, autophagy could contribute to both cell survival and cell death. Besides autophagy, oxidative stress has been shown to induce apoptotic signaling pathways leading to cell death. This is similar to the effect of arsenic trioxide As 2 O 3 on cell death in human T-lymphocytic leukemia and myelodysplastic syndrome MDS cell lines. This indicates that when H 2 O 2 -or 2-ME-induced apoptotic cell death pathway is blocked, cells preferentially die by the autophagic pathway. Conversely, blockage of autophagosome accumulation failed to significantly alter apoptosis. This suggest that apoptosis occurs independent of autophagy. The amount of oxidative stress-induced cell death was not completely blocked by inhibiting either apoptosis or autophagy. This indicates that there is a third cell death pathway. This third type of cell death could be the necrotic cell death pathway. This form of cell death is passive and causes cellular contents to be released to the extracellular space and often causes inflammation. Reports have shown that when apoptotic or autophagic cell death was blocked, necrotic cell death was observed. Nevertheless, our results indicate that autophagic cell death pathway plays an important role in oxidative stress-induced cell death. Autophagy pathways have been extensively studied. A recent report suggests that ROS could be involved in caspase-independent cell death in macrophage cells. Tiron treatment however failed to completely eliminate oxidative stress-induced ROS generation suggesting that higher levels of ROS or the types of ROS generated might be important in regulating autophagy independent of apoptosis. Indeed, ROS have many effects on cells including DNA damage, mitochondrial dysfunction, activation of signaling pathways and activation of transcription factors leading to upregulation of genes. This agrees with the report by Thorpe et al. The mechanism for ROS-mediated upregulation of beclin-1 and the differences between ROS-induced apoptosis and autophagy will be the focus for further investigation. Cancer cells produce higher levels of ROS than normal cells, and this contributes to cancer progression. Hagen et al. Indeed, overexpression of mitochondrial SOD2 blocks ROS generation and autophagosome accumulation induced by 2-ME. A strategy to sensitize cancer cells to drug-induced apoptosis is to combine an ROS-generating drug with the inhibition of mitochondrial respiration enhancing ROS production and cell death such as combining As 2 O 3 with rotenone an inhibitor of mETC complex I. In addition, autophagy failed to be significantly induced in primary astrocytes under oxidative stress. Thus, targeting ROS generation could selectively induce autophagic cell death in cancer cells. In conclusion, oxidative stress induces autophagy and provides a novel mechanism for oxidative stress-induced cell death that is selective toward transformed and cancer cells. This may lead to new strategies to develop therapeutic drugs that will selectively target cancer cells to undergo autophagy-induced cell death independent of apoptosis. Benzyloxycarbonyl-Val-Ala-Asp zVAD fmk, zVAD was purchased from Calbiochem Mississaga, Ontario and dihydroethidium HE from Invitrogen Burlington, Ontario. HE, 2-ME, zVAD and DAPI were dissolved in dimethyl sulfoxide DMSO. AO, EB and trypan blue were dissolved in 1 × PBS. The final concentration of DMSO in media was less than 0. The concentrations of some reagents used in this study were: H 2 O 2 , 1. Beclin-1 and ATG-5 primary antibodies and their secondary antibody donkey anti-goat HRP were purchased from Santa Cruz Biotechnology, Inc. CA, USA. ATG-7 antibodies were purchased from PromoKine Inc. SOD2 antibodies were purchased from StressGen Biotechnologies Victoria, Canada. Rabbit anti-actin antibody was purchased from Sigma, rabbit anti-LC3 antibody from Abgent Inc. The siRNA specific for human beclin-1 was purchased from Dharmacon Lafayette, CO, USA and the sequences used were same as those by Degenhardt et al. TX, USA targeting exons 1 and 2 and sod-2 siRNA was purchased from Ambion Inc. Medium for the stabilized HeLa cells with overexpression of SOD2 was also supplemented with 0. In this study, except otherwise stated, HeLa cells refer to the wild-type cell line. Cell death was analyzed by measuring the permeability of the plasma membrane to AO-EB or trypan blue. Cell suspension was centrifuged in an eppendorf tube. Live cells are permeable to AO but not to EB and stained green, and dead cells permeable to both AO and EB, and EB stains the DNA red. This red staining is distinctive from AO staining of autolysosomes red in the cytoplasm. At least cells were counted for each treatment. Cell death can also be analyzed by staining cells with trypan blue and counting cells under a microscope. Briefly, cells were collected and suspended in 0. Then, cells were stained with trypan blue with a final concentration of 0. Stained cells were analyzed on a flow cytometer using CellQuest software Becton Dickinson, San Jose, CA. Two peaks in the histograph were observed. The first peak represents viable cells, which were dimly fluorescent and not permeable to trypan blue. The second peak represents dead cells, which were brightly fluorescent and permeable to trypan blue. On the fourth day, cells from each big plate were split into six-well plates with same amount of cells in each well. On the fifth day, old media were removed, and fresh media and H 2 O 2 were added. On the sixth or seventh day, cells were collected and analyzed, and partial cells were lysed to make protein lysates for western blot. Transfection of siRNA into cells follows the Invitrogen protocols with some modifications. The cells were washed once with plain DMEM medium. Then, 2. Autophagy is characterized by the formation of AVOs autophagosomes and autolysosomes. The intensity of the red fluorescence is proportional to the degree of acidity. Thus, the formation of AVOs can be quantified. Cells were washed twice with PBS, resuspended in 0. The DNA was stained with antifade DAPI solution after cells were fixed with 3. Apoptosis was analyzed by measuring sub-G1 peaks indication of DNA fragmentation on a flow cytometer after cells were fixed with ethanol and stained with propidium iodide as stated previously. TUNEL assay Roche Inc. that detects DNA breaks was detected on flow cytometer as per the manufacturer's instructions. ROS generation was determined by flow cytometry after cells were stained with HE. First, HE was dissolved in DMSO to make aliquots of stock solution of 1. When used HE is taken out, covered with aluminum foil, and kept on ice until it is melt. For staining of cells, cells were centrifuged down and the pellet was resuspended in 0. glutaraldehyde in 0. The samples were then washed again, dehydrated with graded alcohol, and embedded in Epon-Araldite resin Canemco Inc. Ultrathin sections were cut on a Reichert ultramicrotome, counterstained with 0. Western blot analysis was performed as stated previously. All experiments were repeated at least three times and each experiment was carried out at least by triplicates. The data were expressed as means±S. Statistical analysis was performed by using Student's t test using at least three independent data points. The software used is excel or sigma blot. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest ; : — Article CAS PubMed PubMed Central Google Scholar. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene ; 23 : — Article CAS PubMed Google Scholar. Mariño G, López-Otín C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. CMLS Cell Mol Life Sci ; 61 : — Article PubMed Google Scholar. Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ ; 12 : — Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol ; 6 : — Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res ; 63 : — CAS PubMed Google Scholar. Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene ; 24 : — Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M et al. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell ; 22 : — Ito H, Aoki H, Kuhnel F, Kondo Y, Kubicka S, Wirth T et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst ; 98 : — Xue L, Fletcher GC, Tolkovsky AM. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr Biol ; 11 : — Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem ; : — Matsui Y, Takagi H, Qu X, Adbellatif M, Sakoda H, Asano T et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res ; : — Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J ; 26 : — Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Update ; 7 : 97— Article CAS Google Scholar. Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA ; : — Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell ; 10 : — Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog ; 5 : Article PubMed PubMed Central Google Scholar. Benassi B, Fanciulli M, Fiorentino F, Porrello A, Chiorino G, Loda M et al. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol Cell ; 21 : — Gao N, Rahmani M, Dent P, Grant S. Takeda M, Shirato I, Kobayashi M, Endou H. Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron ; 81 : — Li L, Heldin NE, Grawe J, Ulmsten U, Fu X. Induction of apoptosis or necrosis in human endometrial carcinoma cells by 2-methoxyestradiol. Anticancer Res ; 24 : — Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie ; 84 : — Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting? Autophagy ; 3 ; e-pub ahead of print. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature ; : — Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB et al. The ischemia—reperfusion injury IRI may be characterized as a problematical pathologic condition encompassing numerous factors such as oxidative stress. Extensive damages attributed to mitochondria have been considered in IRI containing unbalanced swelling and crista fragmenting of mitochondria, in ischemic cerebrovascular disease, particularly throughout the acute phase. This impairment motivates mPTP to open unremittingly, leading to the occurrence of an alteration in ROS formation, energy shortage, and membrane potential, thus prompting autophagy. It has been witnessed that autophagy is caused in the mouse striatum and cortex subsequent cerebral hypoxic-ischemia and augmented by a consequent ROS overproduction. Oxidative damage in mouse striatum and cortex subsequent cerebral hypoxia—ischemia causes autophagy. Autophagy during this circumstance can noticeably save neurons in the ischemic condition by inhibiting necrosis and apoptosis via abolishing damaged mitochondria [ ]. It has been conveyed that insufficiency of fatty acids FAs intensely alters ischemia-induced autophagy activation. This is revealed by the raised levels of LC3 and Beclin1 expression, along with significant growth in 8-OHdG, signifying that FAs deficiency can ameliorate the levels of autophagy via induction of oxidative damage. A study has presented that together ROS and autophagy are involved in reperfusion injury afterward cerebral ischemia which antioxidants frequently stimulate autophagy [ ]. The use of antioxidants well-identified as revulsive of autophagy has the ability to attenuate neuronal damage and expressively alleviate the infarcted area. Therefore, we will wonder that antioxidants might play a defensive character in ischemic injury by prompting autophagy. There could also be some more complex crosstalk mechanisms concerning autophagy and oxidative stress in the necessity of additional research [ ]. Furthermore, SIRT3 may be a well-maintained deacetylase connected to biological roles like stress resistance, mitochondrial redox homeostasis, and energy metabolism. Pharmacological or genetic impeding of autophagy can amend SIRT6-mediated neuronal damage, feasibly via mitigating Akt signaling allied with oxidative damage in the OGD model of SH-SY5Y neurons. Besides, moderate activation of ROS can encourage Parkin translocation into the injured mitochondria, afterward suffer Parkin-mediated mitophagy and certify the mitochondria integrity in ischemic brain injury [ ]. So, as a blood flow reduction situation, chronic cerebral hypoperfusion is a problem for treating many debilitating cerebral disorders, leading to apoptotic neuronal cell death and cognitive disorders. Hence, for decreasing apoptotic neuronal cell death, a treatment for chronic cerebral hypoperfusion could be a good idea [ 76 ]. Su and colleagues explored cannabinoid receptor agonist WIN55,—2 WIN and fatty acid amide hydrolase inhibitor URB URB in a rat model to be considered for treating chronic cerebral hypoperfusion induced apoptosis. They found that WIN and URB treated rats showing better action in memory tests and neural cells counting, proteomics, genomics, and brain sections analysis showed a reduction of neurotoxicity, phosphorylation of c-Jun N-terminal kinases JNK , caspase-3 initiation, and Bcl-2 to Bax ratio [ , , ]. In another study by this team, they investigated the URB effect on up-regulated autophagy in chronic cerebral hypoperfusion status in the brains of a rat model of chronic cerebral hypoperfusion; they reported that inhibition of autophagy by URB reduced autophagosomes accumulation and decreased synaptic degradation caused by excessive autophagy, and diminished mitochondrial dysfunction and mitophagy. Based on increasing phosphorylated Akt and mTOR levels and unchanged AMPK, they suggested Akt-mTOR signaling as a possible pathway to inhibit autophagy by URB [ ]. A meta-analysis about the effect of autophagy regulation on spinal cord injury improvement showed it altered neurological recovery, whether it is increased or decreased, but no apparent discrepancy was seen among increment and decrement of autophagy in spinal damage treatment in a rat model [ ]. Zhu et al. discussed that acupuncture could increase mTORC1expression in the peri-infarct cortex and decrease ULK1 , ATG13 , and Beclin1 amounts. However, above and beyond, acupuncture attenuated LC3-II and Beclin 1 expression and the number of autophagosomes [ ]. Transcription factor E3 TFE3 has excellent potential for use in ROS-mediated autophagy dysfunction following spinal cord injury. TFE3 has been regulated partially afterward the spinal cord injury via regulation of AMPK-mTOR and AMPK-SKP2-CARM1 signaling pathways [ 61 ]. The induction of therapeutic autophagy acts as a survival mechanism. However, progressive autophagy, also known as autophagic cell death, can lead to non-apoptotic cell death. Many human diseases such as autoimmune diseases, cancers, cardiovascular disorders, microbial infections, metabolic diseases, inflammatory responses, bone diseases, renal ailments, liver disorders, NDDs, etc. Autophagy can be utilized both in maladaptive and adaptive functions in the pathogenesis of various diseases Fig. A variety of autophagy implications are exhibited in this figure. Khandia and coworkers explained the roles of autophagy in infectious diseases in their recent review article very carefully. Autophagy has a critical role in viral infections, for instance, bird flu, swine fever, Ebola virus disease, Zika virus infection, SARS, Chikungunya infection, Dengue virus infection, viral encephalitis, Crimean-Congo hemorrhagic fever CCHF virus, Hendra virus infection, Nipah virus infection, and the West Nile virus infection [ ]. Autophagosome formation is induced in the early stages of the influenza A virus. However, later stages result in the inhibition of autophagosomal maturation. MTORC1 downregulates classical swine fever virus replication through autophagy and IRES-dependent translation. Transcription of ATGs is promoted in severe acute respiratory syndrome SARS -coronavirus. Moreover, it has been reported that endoplasmic reticulum ER stress in Dengue virus DENV infections led to autophagy activation, viral replication, and pathogenesis. Autophagy has also been shown to have a crucial role in microbial infections, including those caused by Listeria , Salmonella , Shigella , Streptococcus , Mycobacterium tuberculosis , and Salmonella enterica serovar Typhimurium [ ]. The role of autophagy in Toxoplasmacidal mechanisms has been observed [ ]. Autophagy is more involved in cancers than other diseases; the effects of autophagy on cancer are highly dependent on the situation. It can eliminate the tumor or be ineffective or even strengthen the spread of the tumor. In recent years many studies have been conducted to investigate the use of autophagy to treat diseases. However, the critical question of whether autophagy could be regulated for cancer treatment or not remained unanswered, although there are now pieces of evidence that autophagy in cancer acts as a lifeline to chemotherapy. Although, studies have shown that autophagy is a vital cell rescue mechanism for clearing defective proteins and damaged organelles that conserve cell energy and function [ 21 , , ]. Plumbagin, a quinonoid component isolated from Plumbago zeylanica L. The roles of miRNAs in osteosarcoma in recent investigations showed miRNAs like MiR involved in determinant sectors of autophagy gene regulation. Beclin1 , LC3 , Metadherin MTDH , ATG5 genes, and the proteins of LC3 , ATG5 that are closely related to autophagy regulation and anticancer drug resistance, when an encounter with miR downregulate obviously [ , , ]. Lovastatin inhibition of autophagy in glioblastoma cells which were resistant to temozolomide, enhanced efficacy of temozolomide through cessation of autophagy cascade by inhibition of LAMP2 and dynein proteins. Impermanent formation of autolysosomes increased glioblastoma cell death [ , ]. Proof of bufalin-induced autophagy comprised the formation of the acidic vesicular organelles, augmentation of autophagolysosomes, and accumulation of LC3-II. More reports presented that the mechanism of bufalin-induced autophagy connected with ATP depletion involved an increased AMPK activation, diminished phosphorylation of mTOR, and its downstream targets 4EBP1 and p70S6K1 [ ]. Autophagy inhibition in lung cancer can overcome drug resistance, like the effect of candesartan and gingerol on TRAIL-resistant lung cancers via autophagy inhibition caused a reduction of drug resistance [ , ]. A study on the effect of cigarette smoke extract CSE on fibroblast cells of the lung seen CSE induction of autophagy and related proteins, such as optineurin. Inhibition optineurin reversed the pathway and reduced p62 protein and IL-8 expression in these treated fibroblasts. Zhang and colleagues explored the linkage of autophagy to the effectiveness of chemotherapy with a regimen of 5-FU and 3-MA in the treatment of Squamous cell carcinoma SCC. SCC is a type of malignant skin cancer, and its prevalence is one-fifth of non-melanoma skin cancers. Yi Zhang and colleagues explored the effect of sodium selenite on leukemia. They found p53 was a primary up regulator of phospholipid scramblase 1 PLSCR1 , acting as a confluence shifting autophagy to apoptosis in leukemia. PLSCR1 Tuning of apoptosis and autophagy possibly is the primary mechanism in the efficacy of chemotherapy [ 76 , ]. Ectopic expression of Beclin1 in breast cancer cells MCF-7 activated autophagy. Moreover, the monoallelic deletion of Beclin1 was witnessed in numerous specimens of human breast, ovarian, and prostate cancer cells [ ]. UVRAG, an upregulating agent of the Beclin1-class III PI3K complex, suppressed the proliferation and tumorigenicity of colon cancer cells. In addition, knockout of Bif-1which interacted with Beclin1 and activated the Beclin1-class III PI3K complex, ameliorating spontaneous tumors' progression in vivo [ , , ]. A systemic mosaic deletion of ATG5 or a liver-specific ATG7 deficiency in mice led to the development of benign liver tumors, proposing the critical role of autophagy in the abrogation of spontaneous tumor genesis [ ]. Autophagy decreases during the aging process. It seems that maintaining autophagy is an effective anti-aging treatment. During the aging process, the innate immune system response to external antigen decreases, ROS production and IL-1B concentration, and IL Increase by macrophages. Other innate immune cells that are affected by aging are neutrophils. Although there is no reduction in the total number of these cells during aging, studies have shown that their capacity for phagocytosis and xenophagy have an inverse relationship with age. They find similar conditions diminish antigens presentation and chemotaxis. In addition to the weakening of immunity against pathogens, chronic inflammation develops, and immune cells do not return to the basal level of homeostasis after inflammation. Peripheral cells such as adipose increase inflammatory status by increasing the release of cytokines. Cell-mediated immunity because of DNA damages and shortening of telomeres have obstacles to replicating and reproducing. Their ability to create a memory is reduced [ , ]. T cells secrete proinflammatory cytokines without antigen stimulation, do not respond to apoptotic signals, diminished the production of the central cytokines like TNF-α, IL-2, INF-γ, and granzyme B, and consequently, their response to infections decreases. Although B lymphocytes are less studied in aging research, in some studies on human vaccination, due to reduced humoral immune response, it appears to be related to B cells malfunction [ 76 , ]. Furthermore, macrophages, B, and T cells for phagocytosis, antigen presentation, and hemostasis require autophagy. Therefore, it is conceivable to reduce the immunological effects of aging by targeting autophagy [ ]. Recent studies indicated that AMPK activation inhibited cell-induced aging by boosting activation of autophagy, detention of mTORC1 process, and reducing oxidative stress. Hence, it seems to be an excellent strategy to enable AMPK to stop cell aging [ , ]. Moreover, the ATG5 gene has participated in the incidence and development of systemic lupus erythematosus as an autoimmune disease [ ]. Additionally, autophagy and chronic inflammation involved cystic fibrosis, an incurable genetic condition initiated by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator. Meng and colleagues claimed autophagy could mitigate pulmonary fibrosis by mediating the activation of the NOD-like receptor family pyrin domain containing 3 NLRP3 inflammasome induced by angiotensin II-mediated ROS via modulation of redox balance [ ]. Moreover, asthma and COPD can be related to the role of autophagy [ ]. Evidence indicated that autophagy plays a central role in tissue remodeling. The changed autophagy pathway in response to cellular stress in asthma and COPD resulted in activation and crosstalk between structural airway and immune cells. This further leads to autophagy impairment causing intracellular constituents degradation and secreting inflammation mediators, which cause lung airway remodeling [ ]. Corneal oxidative stress upregulated LC3 , Beclin 1 , and ATG12 levels; however, P62 were downregulated. Hence, various signaling pathways, such as the mTOR, showed the interplay of ROS and autophagy in corneas [ ]. Regulation of the expression of LC3II and P62 regarding suppression of the senescence of lens epithelial cells by restoring autophagy flux after metformin administration was observed. More studies about the role of autophagy in glaucoma have been reported [ ]. Autophagy can act as a cyto-protector or a cyto-killer in glaucoma. The dual role of autophagy in glaucoma progression is related to the glaucoma stage, autophagy stage, autophagy detection time, and even genomic changes [ ]. Cardiometabolic disease comprises a wide range of cardiovascular diseases, diabetes, and obesity [ ]. In vitro and in vivo results elucidated that hindering the expression of ATG7 had a defective role regarding insulin signaling and augmented ER stress. On the contrary, reinstatement of ATG7 expression in the liver limited ER stress and improved insulin activity and systemic glucose tolerance in mice with obesity. Furthermore, autophagy has a crucial role in normal adipogenesis, and abridge of autophagy has anti-obesity and insulin-sensitizing properties [ , ]. According to the shreds of evidence, impaired autophagy might contribute to insulin deficiency and hyperglycemia. Autophagy was considered a significant regulator in pancreatic β-cells, and ATG7 mutation exhibited damaged glucose tolerance. In an investigation on the effect of liraglutide on cardiac fibrosis caused by aortic banding in rats, results indicated that inhibition of p70S6K , a ribosomal protein S6 kinase, and mTOR signaling by liraglutide, showing a positive effect on reduction of heart dysfunction emanating from fibrosis and cardiomyocyte hypertrophy by increase autophagy [ ]. ATG5 deficiency was reported to contribute to various cardiovascular diseases [ ]. Dysregulation of autophagy is one of the reasons responsible for the pathogenesis of acute kidney injury, or incomplete kidney repair after acute kidney injury and chronic kidney disease of diverse aetiologies, comprising diabetic kidney disease, focal segmental glomerulosclerosis, and polycystic kidney disease. Autophagy also plays a critical role in kidney aging. CMA, AMPK, sirtuins, and the mTOR pathway are considered the most feasible gates for regulating autophagy-related to renal disease [ 11 , , ]. Autophagy has an emerging primary function in maintaining the balance of liver metabolism and the modulation of its physiology. On the contrary, numerous documents have shown that autophagy may disease-dependently play a part in the pathogenesis of liver disorders, like Wilson's disease, α-1 antitrypsin deficiency, hepatocellular carcinoma, cirrhosis, acute liver injury, chronic alcohol-associated liver disease, liver hepatitis, fibrosis, steatosis, and non-alcoholic fatty liver disease NAFLD [ ]. Carbamazepine, rapamycin, and ursodeoxycholic acid are drugs with autophagy induction and assistive in combating various liver diseases [ ]. There are a number of transcription factors that can assist in regulating hepatic functions related to autophagy. These transcription factors are encompassing CREB, Nrf2, PPARα, and TFEB. Ueno et al. demonstrated that the mTORC1 complex acts as a fundamental portion of the liver's amendment to metabolic disorders, containing nutrient starvation. Inactivation of mTORC1 causes TFEB activation and NCOR1 inactivation. Moreover, the integral functioning of Nrf2 and Parkin-mediated mitophagy have participated in the modulation of autophagy in liver disorders [ , , , ]. Autophagic catabolism is involved in controlling and managing the survival and working of osteoclasts, osteocytes, and osteoblasts. Hence it is essential for the conservation of skeletal homeostasis. Unusual autophagic action causes obviation of the balance of the bone-remodeling manifesting as pathological conditions, comprising osteopetrosis and osteoporosis. Regulation of autophagy has eliminated therapeutic potential in the treatment and prevention of bone-related diseases. According to the pivotal character of skeletal muscles in metabolism control, maintaining the muscle homeostasis through making balance among catabolic and anabolic processes is of high emphasis. Hence, autophagy is a principle catabolic mechanism in the skeletal muscles which helps in the achievement of the mentioned aim. The significance of autophagy as a helpful target and recommend explaining associations between protein unfurling and mTOR-dependent or mTOR-independent hypertrophic reactions is likely to uncover particular helpful windows for treating muscle squandering clutters muscle function loss or paralysis [ 19 , ]. While the appropriate function of autophagy is vital for the exact function and CNS development, any single gene disorder related to autophagy signaling can be harmful. In summary, regarding the emerging emphasis on cellular autophagy, a comprehensive literature overview was conducted to discuss the pharmacological aspects of autophagy, focusing on its interplay with oxidative stress in neurological disorders. Various human diseases have been contributed to alterations in autophagy entailing cancers, cardiometabolic diseases, renal and liver diseases, bone diseases, neurological disease, aging, and immune dysfunctions. This interplay assists in the foundation of variable targets for drug discovery. Wang M-M, Feng Y-S, Yang S-D, Xing Y, Zhang J, Dong F, et al. The relationship between autophagy and brain plasticity in neurological diseases. Front Cell Neurosci. CAS PubMed PubMed Central Google Scholar. Farkhondeh T, Samarghandian S, Pourbagher-Shahri AM, Sedaghat M. J Cell Physiol. CAS PubMed Google Scholar. Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. Wang S-Y, Yu Q-J, Zhang R-D, Liu B. Int J Biochem Cell Biol. Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metabol. CAS Google Scholar. Rahman MA, Rahman MR, Zaman T, Uddin MS, Islam R, Abdel-Daim MM, et al. Emerging potential of naturally occurring autophagy modulators against neurodegeneration. Curr Pharm Des. Kesidou E, Lagoudaki R, Touloumi O, Poulatsidou K-N, Simeonidou C. Autophagy and neurodegenerative disorders. Neural Regen Res. Batatinha HAP, Diniz TA, de Souza Teixeira AA, Krüger K, Rosa-Neto JC. Regulation of autophagy as a therapy for immunosenescence-driven cancer and neurodegenerative diseases: The role of exercise. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Tang C, Livingston MJ, Liu Z, Dong Z. Autophagy in kidney homeostasis and disease. Nat Rev Nephrol. Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. Yun HR, Jo YH, Kim J, Shin Y, Kim SS, Choi TG. Roles of autophagy in oxidative stress. Int J Mol Sci. CAS PubMed Central Google Scholar. Hajzadeh MA, Rajaei Z, Shafiee S, Alavinejhad A, Samarghandian S, Ahmadi M. Effect of barberry fruit berberis vulgaris o serum glucose ad lipids i streptozotoci-diabetic rats. Pharmacol Online. Google Scholar. Samarghandian S, Samini F, Azimi-Nezhad M, Farkhondeh T. Anti-oxidative effects of safranal on immobilization-induced oxidative damage in rat brain. Neurosci Lett. doi: Article Google Scholar. Zhu J, Dagda RK, Chu CT. Monitoring mitophagy in neuronal cell cultures. In: Manfredi G, Kawamata H, editors. Cham: Springer; Singh SS, Vats S, Chia AY-Q, Tan TZ, Deng S, Ong MS, et al. Dual role of autophagy in hallmarks of cancer. Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. Grumati P, Bonaldo P. Autophagy in skeletal muscle homeostasis and in muscular dystrophies. PubMed PubMed Central Google Scholar. Desideri E, Vegliante R, Cardaci S, Nepravishta R, Paci M, Ciriolo MR. Du J, Liang Y, Xu F, Sun B, Wang Z. J Pharm Pharmacol. Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian S. Modulatory effects of statins on the autophagy: a therapeutic perspective. Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother Res. Ashrafizadeh M, Zarrabi A, Orouei S, Hushmandi K, Hakimi A, Zabolian A, et al. Eur J Pharmacol. Article PubMed Google Scholar. Desai S, Juncker M, Kim C. Regulation of mitophagy by the ubiquitin pathway in neurodegenerative diseases. Exp Biol Med. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. Talebi M, Mojab F. Int Pharm Acta. Talebi M, İlgün S, Ebrahimi V, Talebi M, Farkhondeh T, Ebrahimi H, et al. Biomed Pharmacother. Talebi M, Kakouri E, Talebi M, Tarantilis PA, Farkhondeh T, İlgün S, et al. Expert Rev Neurother. Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Fransen M, Lismont C. Redox signaling from and to peroxisomes: progress, challenges, and prospects. Talebi M, Talebi M, Kakouri E, Farkhondeh T, Pourbagher-Shahri AM, Tarantilis PA, et al. Tantalizing role of p53 molecular pathways and its coherent medications in neurodegenerative diseases. Int J Biol Macromol. Lan A-P, Chen J, Zhao Y, Chai Z, Hu Y. Neuromolecular Med. PubMed Google Scholar. Lipton JO, Sahin M. The neurology of mTOR. Alexander A, Cai S-L, Kim J, Nanez A, Sahin M, MacLean KH, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci. Kongara S, Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front Oncol. Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species ROS : implications for cancer progression and treatment. Samarghandian S, Azimi-Nezhad M, Farkhondeh T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. Talebi M, Talebi M, Farkhondeh T, Mishra G, İlgün S, Samarghandian S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Pajares M, Jiménez-Moreno N, García-Yagüe ÁJ, Escoll M, de Ceballos ML, Van Leuven F, et al. Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling. Int J Mol Med. Dong X-X, Wang Y, Qin Z-H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. Funderburk SF, Marcellino BK, Yue Z. Mt Sinai J Med. Essick EE, Sam F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev. Cheng W-T, Guo Z-X, Lin C-A, Lin M-Y, Tung L-C, Fang K. Oxidative stress promotes autophagic cell death in human neuroblastoma cells with ectopic transfer of mitochondrial PPP2R2B Bbeta2. BMC Cell Biol. Article PubMed PubMed Central Google Scholar. Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. Talebi M, Talebi M, Samarghandian S. Biointerface Res Appl Chem. Uddin MS, Kabir MT, Tewari D, Mamun AA, Mathew B, Aleya L, et al. J Neurol Sci. Talebi M, Esmaeeli H, Talebi M, Farkhondeh T, Samarghandian S. Curr Pharm Biotechnol. Duncan RS, Song B, Koulen P. PubMed Central Google Scholar. Uddin M, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, et al. Front Aging Neurosci. Gorantla NV, Chinnathambi S. Cell Mol Neurobiol. Di Domenico F, Barone E, Perluigi M, Butterfield DA. Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. Uddin MS, Rahman MA, Kabir MT, Behl T, Mathew B, Perveen A, et al. IUBMB Life. Huang J-L, Su M, Wu D-P. Ageing Res Rev. Kim J, Yoon H, Kim J. Curr Enzym Inhib. Du F, Yu Q, Yan S, Hu G, Lue L-F, Walker DG, et al. CNS Drugs. Zheng X, Wang W, Liu R, Huang H, Zhang R, Sun L. Agrawal I, Jha S. Zhang Y-d, Zhao J-j. DNA Cell Biol. Omata Y, Lim Y-M, Akao Y, Tsuda L. Am J Neurodegener Dis. Li L-H, Peng W-N, Deng Y, Li J-J, Tian X-R. Moon J-H, Jeong J-K, Hong J-M, Seol J-W, Park S-Y. Inhibition of autophagy by captopril attenuates prion peptide-mediated neuronal apoptosis via AMPK activation. Mol Neurobiol. Cai Z, Yan L-J, Li K, Quazi SH, Zhao B. Weng M-H, Chen S-Y, Li Z-Y, Yen G-C. Free Radic Biol Med. Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, et al. Histol Histopathol. Ludtmann MHR, Abramov AY. Jankovic J, Tan EK. J Neurol Neurosurg Psychiatry Res. Inamdar NN, Arulmozhi DK, Tandon A, Bodhankar SL. Curr Neuropharmacol. Dagda RK, Zhu J, Chu CT. Lu J, Wu M, Yue Z. Autophagy: biology and diseases. Butler D, Bahr BA. Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Chatta GS, Price TH, Stratton JR, Dale DC. Aging and marrow neutrophil reserves. J Am Geriatr Soc. Zhuang X-X, Wang S-F, Tan Y, Song J-X, Zhu Z, Wang Z-Y, et al. Cell Death Dis. Ning B, Zhang Q, Wang N, Deng M, Fang Y. Neurochem Res. Oh SE, Mouradian MM. Regulation of signal transduction by DJ Ebert AD, Beres AJ, Barber AE, Svendsen CN. Exp Neurol. Wang X-W, Yuan L-J, Yang Y, Zhang M, Chen W-F. Am J Physiol Endocrinol Metab. Zhu J, Gao W, Shan X, Wang C, Wang H, Shao Z, et al. Brain Res. Zhang L, Zhang L, Li L, Hölscher C. Mao K, Chen J, Yu H, Li H, Ren Y, Wu X, et al. Aging Cell. Lin C-H, Wei P-C, Chen C-M, Huang Y-T, Lin J-L, Lo Y-S, et al. Guo Y-L, Duan W-J, Lu D-H, Ma X-H, Li X-X, Li Z, et al. He X-H, Lin F, Qin Z-H. Current understanding on the pathogenesis of polyglutamine diseases. Neurosci Bull. Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RAV, et al. Sci Transl Med. Cortes CJ, La Spada AR. Drug Discov Today. An T, Shi P, Duan W, Zhang S, Yuan P, Li Z, et al. Oxidative stress and autophagic alteration in brainstem of SOD1-G93A mouse model of ALS. Deng Z, Sheehan P, Chen S, Yue Z. Mol Neurodegener. Ma L, Herren AW, Espinal G, Randol J, McLaughlin B, Martinez-Cerdeño V, et al. Acta Neuropathol Commun. Wardman JH, Henriksen EE, Marthaler AG, Nielsen JE, Nielsen TT. Enhancement of autophagy and solubilization of ataxin-2 alleviate apoptosis in spinocerebellar ataxia type 2 patient cells. Morani F, Doccini S, Sirica R, Paterno M, Pezzini F, Ricca I, et al. Functional transcriptome analysis in ARSACS KO cell model reveals a role of sacsin in autophagy. Sci Rep. Ye B, Wang Q, Hu H, Shen Y, Fan C, Chen P, et al. Restoring autophagic flux attenuates cochlear spiral ganglion neuron degeneration by promoting TFEB nuclear translocation via inhibiting MTOR. Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. Yamada D, Kawabe K, Tosa I, Tsukamoto S, Nakazato R, Kou M, et al. Inhibition of the glutamine transporter SNAT1 confers neuroprotection in mice by modulating the mTOR-autophagy system. Commun Biol. Mackenzie B, Schäfer MKH, Erickson JD, Hediger MA, Weihe E, Varoqui H. Functional properties and cellular distribution of the system A glutamine transporter SNAT1 support specialized roles in central neurons. J Biol Chem. Hägglund MGA, Sreedharan S, Nilsson VCO, Shaik JHA, Almkvist IM, Bäcklin S, et al. Identification of SLC38A7 SNAT7 protein as a glutamine transporter expressed in neurons. Bagchi S, Baomar HA, Al-Walai S, Al-Sadi S, Fredriksson R. Histological analysis of SLC38A6 SNAT6 expression in mouse brain shows selective expression in excitatory neurons with high expression in the synapses. PLoS ONE. Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, et al. Tsc1 hamartin confers neuroprotection against ischemia by inducing autophagy. Nat Med. Liu Y, Xue X, Zhang H, Che X, Luo J, Wang P, et al. Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Aredia F, Scovassi AI. A new function for miRNAs as regulators of autophagy. Future Med Chem. Wang Y, Meng C, Zhang J, Wu J, Zhao J. Int Immunopharmacol. Fang C, Gu L, Smerin D, Mao S, Xiong X. The interrelation between reactive oxygen species and autophagy in neurological disorders. Liu S, Su Y, Sun B, Hao R, Pan S, Gao X, et al. Su SH, Wang YQ, Wu YF, Wang DP, Lin Q, Hai J. Behav Brain Res. Su SH, Wu YF, Lin Q, Yu F, Hai J. Cannabinoid receptor agonist WIN55,—2 and fatty acid amide hydrolase inhibitor URB suppress chronic cerebral hypoperfusion-induced neuronal apoptosis by inhibiting c-Jun N-terminal kinase signaling. Su SH, Wu YF, Lin Q, Hai J. Cannabinoid receptor agonist WIN55,—2 and fatty acid amide hydrolase inhibitor URB ameliorate neuroinflammatory responses in chronic cerebral hypoperfusion model by blocking NF-κB pathways. Naunyn Schmiedebergs Arch Pharmacol. Wang D, Lin Q, Su S, Liu K, Wu Y, Hai J. URB improves cognitive impairment induced by chronic cerebral hypoperfusion by inhibiting mTOR-dependent autophagy. Zhang D, Zhu D, Wang F, Zhu J-C, Zhai X, Yuan Y, et al. Therapeutic effect of regulating autophagy in spinal cord injury: a network meta-analysis of direct and indirect comparisons. Zhu Y, Tang Q, Wang G, Han R. Tanshinone IIA protects hippocampal neuronal cells from reactive oxygen species through changes in autophagy and activation of phosphatidylinositol 3-kinase, protein kinas B, and mechanistic target of rapamycin pathways. Curr Neurovasc Res. Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: a comprehensive review. Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Žugich J. Key role of T cell defects in age-related vulnerability to West Nile virusT cell defects and age-related vulnerability to WNV. J Exp Med. Khandia R, Dadar M, Munjal A, Dhama K, Karthik K, Tiwari R, et al. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: current knowledge and prospects for disease prevention, novel drug design, and therapy. Krishnamurthy S, Konstantinou EK, Young LH, Gold DA, Saeij JPJ. The human immune response to Toxoplasma: autophagy versus cell death. PLoS Pathog. Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Jiang G-M, Tan Y, Wang H, Peng L, Chen H-T, Meng X-J, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. Sun C-Y, Zhang Q-Y, Zheng G-J, Feng B. Autophagy and its potent modulators from phytochemicals in cancer treatment. Cancer Chemother Pharmacol. Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Role of autophagy in osteosarcoma. J Bone Oncol. Wang P, Zhao ZQ, Guo SB, Yang TY, Chang ZQ, Li DH, et al. Roles of microRNA in suppressing proliferation and promoting sensitivity of osteosarcoma cells via metadherin-mediated autophagy. Orthop Surg. Jamali Z, Taheri-Anganeh M, Shabaninejad Z, Keshavarzi A, Taghizadeh H, Razavi ZS, et al. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. Zhu Z, Zhang P, Li N, Kiang KMY, Cheng SY, Wong VKW, et al. Lovastatin enhances cytotoxicity of temozolomide via impairing autophagic flux in glioblastoma cells. Biomed Res Int. Mora R, Régnier-Vigouroux A. Shen S, Zhang Y, Wang Z, Zhang R, Gong X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int J Biol Sci. Nazim UM, Jeong J-K, Seol J-W, Hur J, Eo S-K, Lee J-H, et al. Inhibition of the autophagy flux by gingerol enhances TRAIL-induced tumor cell death. Oncol Rep. Rasheduzzaman M, Park S-Y. Antihypertensive drug-candesartan attenuates TRAIL resistance in human lung cancer via AMPK-mediated inhibition of autophagy flux. Exp Cell Res. Hou HH, Pan HJ, Liao WY, Lee CH, Yu CJ. Autophagy in fibroblasts induced by cigarette smoke extract promotes invasion in lung cancer cells. Int J Cancer. Zhang L, Zhang J, Chen L, Wang J. Autophagy in human skin squamous cell carcinoma: Inhibition by 3-MA enhances the effect of 5-FU-induced chemotherapy sensitivity. Zhang L, Ji Z, Zhang J, Yang S. Photodynamic therapy enhances skin cancer chemotherapy effects through autophagy regulation. Photodiagnosis Photodyn Ther. Zhang Y, Du Y, Le W, Wang K, Kieffer N, Zhang J. Redox control of the survival of healthy and diseased cells. Mancias JD, Kimmelman AC. Mechanisms of selective autophagy in normal physiology and cancer. J Mol Biol. Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. Yun CW, Lee SH. The roles of autophagy in cancer. Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. Hazeldine J, Lord JM, Hampson P. Immunesenescence and inflammaging: a contributory factor in the poor outcome of the geriatric trauma patient. Solana R, Pawelec G, Tarazona R. Aging and Innate Immunity. Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, et al. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol. Lin S-C, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Pourbagher-Shahri AM, Farkhondeh T, Talebi M, Kopustinskiene DM, Samarghandian S, Bernatoniene J. An overview of NO signaling pathways in aging. Lin X-T, Zheng X-B, Fan D-J, Yao Q-Q, Hu J-C, Lian L, et al. Inflamm Bowel Dis. Kato H, Perl A. Blockade of treg cell differentiation and function by the interleukin—mechanistic target of rapamycin axis via suppression of autophagy in patients with systemic lupus erythematosus. Arthritis Rheumatol. Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Xie W, Zhou J. Aberrant regulation of autophagy in mammalian diseases. Biol Lett. Meng Y, Pan M, Zheng B, Chen Y, Li W, Yang Q, et al. Autophagy attenuates angiotensin II-induced pulmonary fibrosis by inhibiting redox imbalance-mediated NOD-like receptor family pyrin domain containing 3 inflammasome activation. Yin Y, Zong R, Bao X, Zheng X, Cui H, Liu Z, et al. Oxidative stress suppresses cellular autophagy in corneal epithelium. Invest Ophthalmol Vis Sci. Farkhondeh T, Samarghandian S, Azimi-Nezhad M. The role of arsenic in obesity and diabetes. Qian Q, Zhang Z, Orwig A, Chen S, Ding W-X, Xu Y, et al. S-Nitrosoglutathione Reductase Dysfunction Contributes to Obesity-Associated Hepatic Insulin Resistance via Regulating Autophagy. Zheng R-H, Zhang W-W, Ji Y-N, Bai X-J, Yan C-P, Wang J, et al. Lenoir O, Tharaux P-L, Huber TB. Autophagy in kidney disease and aging: lessons from rodent models. Kidney Int. Allaire M, Rautou P-E, Codogno P, Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol. Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. Yazdani E, Talebi M, Zarshenas M, Moein M. Evaluation of possible antioxidant activities of barberry solid formulation, a selected formulation from Traditional Persian Medicine TPM via various procedures. Ke P-Y. Diverse functions of autophagy in liver physiology and liver diseases. Int J Mol Med Sci. Yin X, Zhou C, Li J, Liu R, Shi B, Yuan Q, et al. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. Yang Y, Klionsky DJ. Autophagy and disease: unanswered questions. Cell Death Differ. Ebrahimi-Fakhari D, Saffari A, Wahlster L, Lu J, Byrne S, Hoffmann GF, et al. |
| Human Verification | Dev Cell — Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature — ubiquitin protease. Nat Cell Biol 10 5 — Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T The role of autophagy during the early neonatal starvation period. Essays Biochem — Lapaquette P, Guzzo J, Bretillon L, Bringer MA Cellular and molecular connections between autophagy and inflammation. Mediat Inflamm Laplante M, Sabatini DM mTOR signaling in growth control and disease. Cell — Mathew R, Karantza-Wadsworth V, White E Role of autophagy in cancer. Nat Rev Cancer — Mijaljica D, Prescot M, Devenish RJ The intricacy of nuclear membrane dynamics during nucleophagy. Nucleus — Mizushima N Autophagy: process and function. Nakatogawa H Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy. Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K The role of autophagy in the heart. Nixon RA Autophagy, Amyloidogenesis and Alzheimer disease. Onodera J, Ohsumi Y Autophagy is required for maintenance of aminoacid levels and protein synthesis under nitrogen starvation. Otomo C, Metlagel Z, Takaesu G, Otomo T Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat Struct Mol Biol — Overbye A, Fengsrud M, Seglen PO Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. J Mol Biol — Pattingre S, Tassa A, Qu X, Garuti R, Linag XH, Mizushima N Bcl-2 anti-apoptotic proteins inhibit Beclin 1-dependent autophagy. Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M Interplay between ROSand autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol — Polson HE, De Lartigue J, Rigden DJ, Reedijk M, Urbe S, Clague MJ, Tooze SA Mammalian Atg18 WIPI2 localizes to omegasome-anchoredphagophores and positively regulates LC3 lipidation. Qian M, Fang X, Wang X Autophagy and inflammation. Clin Transl Med — Qu X, Yu J, Bhagat G Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. Quan W, Lim YM, Lee MS Role of autophagy in diabetes and endoplasmic reticulum stress of pancreatic β-cells. Exp Mol Med — Ravikumar B, Stewart A, Kita H, Kato K, Duden R, Rubinsztein DC Raised intracellular glucose concentrations reduce aggregation and cell death caused by mutant huntingtin exon 1 by decreasing mTOR phosphorylation and inducing autophagy. Hum Mol Genet — Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Sato M, Sato K Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Satoo K, Noda NN, Kumeta H, Fujioka Y, Mizushima N, Ohsumi Y, Inagaki F The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J — Shiplka T, Elazan Z Essential role of Atg8 isoform LC3C in xenophagy. Siciliano G, Carlesi C, Pasquali L, Piazza S, Pietracupa S, Fornai F Clinical trials for neuroprotection in ALS. CNS Neurol Disord Drug Targets — Simonsen A, Birkeland HC, Gillooly DJ, Mizushima N, Kuma A, Yoshimori T, Slagsvold T, Brech A, Stenmark HA A novel FYVE-domain-containing protein associated with protein granules and autophagic membranes. Singh SB, Davis AS, Taylor GA, Deretic V Human IRGM induces autophagy to eliminate intracellular mycobacteria. Singh R, Kaushik S, Wang Y Autophagy regulates lipid metabolism. Sou YS, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T, Sawada N, Yamada A, Mizushima N, Uchiyama Y The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell — Suzuki SW, Onodera J, Ohsumi Y Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction. PLoS One 6:e Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N Autophagy is essential for preimplantation development of mouse embryos. Vabulas RM, Hartl FU Protein synthesis upon acute nutrient restriction relies on proteasome function. Vaccaro MI Zymophagy: selective autophagy of secretory granules. Int J Cell Biol Viret C, Faure M Regulation of Syntaxin 17 during autophagosome maturation. Trends Cell Biol —3. Viret C, Rozieres A, Faure M Novel insights into NDP52 autophagy receptor functioning. Trends Cell Biol — Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. Weidberg H, Shvets E, Elazar Z Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem — WildP FH, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F Termination of autophagy and reformation of lysosomes regulated by mTOR. Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, Kleinert R, Prinz M, Aguzzi A, Denk H p62 is a common component of cytoplasmic inclusions in protein aggregation diseases. Am J Pathol — Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin1—phosphatidylinositol 3-kinase complex. Download references. Department of Physiology, Kalpana Chawla GMC, Karnal, Haryana, India. Intern, Faculty of Medicine and Health Sciences, SGT University, Gurugram, Haryana, India. You can also search for this author in PubMed Google Scholar. Department of Biochemistry and Biophysics, University of Kalyani, Kalyani, West Bengal, India. Chair and Department of Toxicology, Jagiellonian University Collegium Medicum, Kraków, Poland. Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, UK. University of Burdwan, Bardhaman, West Bengal, India. Institute of Veterinary Physiology, University of Zurich, Zurich, Switzerland. Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, USA. Reprints and permissions. Sethi, J. Oxidative Stress-Induced Autophagy. In: Chakraborti, S. eds Handbook of Oxidative Stress in Cancer: Therapeutic Aspects. Springer, Singapore. Published : 29 September Publisher Name : Springer, Singapore. Print ISBN : Online ISBN : eBook Packages : Biomedical and Life Sciences Reference Module Biomedical and Life Sciences. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Keywords Autophagy Cancer Oxidative stress Reactive oxygen species Neurodegeneration. Buying options Chapter EUR eBook EUR Hardcover Book EUR Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions. References Al Rawi S, Louvet-Vallee S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V Postfertilization autophagy of sperm organelles prevent paternal mitochondrial DNA transmission. Mol Cell Biol —11 CAS Google Scholar Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol — CAS Google Scholar Backer JM The regulation and function of Class III PI3Ks: novel roles forVps Biochem J —17 CAS Google Scholar Barlow AD, Thomas DC Autophagy in diabetes: β-cell dysfunction, insulin resistance, and complications. DNA Cell Biol — CAS Google Scholar Boya P, Reggiori F, Codogno P Emerging regulation and functions of autophagy. Nat Cell Biol — CAS Google Scholar Carlsson SR, Simonsen A Membrane dynamics in autophagosome biogenesis. J Cell Sci — CAS Google Scholar Daniel J, Klionsky DJ, Cuervo AM, Segle PO Methods for monitoring autophagy from yeast to human. Autophagy 3 3 — Google Scholar Deretic V, Levine B Autophagy balances inflammation in innate immunity. Autophagy — CAS Google Scholar Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol — Google Scholar Djavaheri-Mergny M, Amelotti M, Mathieu J NF- kappa B activation represses tumor necrosis factor-alpha-induced autophagy. Biochim Et Biophys Acta — CAS Google Scholar Fortun J, Dunn WA Jr, Joy S, Li J, Notterpek L Emerging role for autophagy in the removal of aggresomes in Schwann cells. J Neurosci — CAS Google Scholar Gatica D, Chiong M, Lavandero S, Klionsky DJ Molecular mechanisms of autophagy in the cardiovascular system. Circ Res — CAS Google Scholar Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev — CAS Google Scholar Harris J Autophagy and cytokines. Cytokine — CAS Google Scholar Hartleben B, Gödel M, Schwesinger CM, Liu S, Ulrich T, Köbler S Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest — CAS Google Scholar Henderson P, Stevens C The role of autophagy in Crohns disease. Cells — Google Scholar Iida T, Onodera K, Nakase H Role of autophagy in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 23 11 — CAS Google Scholar Isakson P, Holland P, Simonsen A The role of ALFY in selective autophagy. Cell Death Differ —20 CAS Google Scholar Itakura E, Mizushima N Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy — CAS Google Scholar Jiang S, Wells CD, Roach PJ Starch-binding domain-containing protein 1 Stbd1 and glycogen metabolism: identification of the Atg8 family interacting motif AIM in Stbd1 required for interaction with GABARAPL1. Biol Chem Res Commun — CAS Google Scholar Johansen T, Lamark T Selective autophagy mediated by autophagic adapter proteins. Autophagy — CAS Google Scholar Jung HS, Lee MS Role of autophagy in diabetes and mitochondria. Leucine induced dephosphorylation of Sestrin2 promotes mTORC1 activation. Cell Signal. Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Shin BY, Jin SH, Cho IJ, Ki SH. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic Biol Med. Shi X, Doycheva DM, Xu L, Tang J, Yan M, Zhang JH. Sestrin2 induced by hypoxia inducible factor1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiol Dis. Zhang XY, Wu XQ, Deng R, Sun T, Feng GK, Zhu XF. Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Kim H, An S, Ro SH, Teixeira F, Park GJ, Kim C, Cho CS, Kim JS, Jakob U, Lee JH, et al. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat Commun. Cordani M, Sanchez-Alvarez M, Strippoli R, Bazhin AV, Donadelli M. Sestrins at the interface of ROS control and autophagy regulation in health and disease. Lim J, Lachenmayer ML, Wu S, Liu W, Kundu M, Wang R, Komatsu M, Oh YJ, Zhao Y, Yue Z. PLoS Genet. Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Orenstein SJ, Cuervo AM. Chaperone-mediated autophagy: molecular mechanisms and physiological relevance. Semin Cell Dev Biol. Zhang L, Wang H, Xu J, Zhu J, Ding K. Toxicol Lett. Zhang J, Kim J, Alexander A, Cai S, Tripathi DN, Dere R, Tee AR, Tait-Mulder J, Di Nardo A, Han JM, et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. TFEB links autophagy to lysosomal biogenesis. Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci USA. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. Gao Q. Oxidative stress and autophagy. Adv Exp Med Biol. Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. Ribas V, Garcia-Ruiz C, Fernandez-Checa JC. Glutathione and mitochondria. Front Pharmacol. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. Yang S, Xia C, Li S, Du L, Zhang L, Zhou R. Defective mitophagy driven by dysregulation of rheb and KIF5B contributes to mitochondrial reactive oxygen species ROS -induced nod-like receptor 3 NLRP3 dependent proinflammatory response and aggravates lipotoxicity. Redox Biol. Kurihara Y, Kanki T, Aoki Y, Hirota Y, Saigusa T, Uchiumi T, Kang D. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J Biol Chem. Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. Ge P, Dawson VL, Dawson TM. Mol Neurodegener. Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Rose CM, Isasa M, Ordureau A, Prado MA, Beausoleil SA, Jedrychowski MP, Finley DJ, Harper JW, Gygi SP. Highly multiplexed quantitative mass spectrometry analysis of ubiquitylomes. Cell Syst. Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. Eiyama A, Okamoto K. Xiao B, Deng X, Lim GGY, Xie S, Zhou ZD, Lim KL, Tan EK. Cell Death Dis. Xiao B, Goh JY, Xiao L, Xian H, Lim KL, Liou YC. Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIFdependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. Bonekamp NA, Volkl A, Fahimi HD, Schrader M. Reactive oxygen species and peroxisomes: struggling for balance. Fransen M, Nordgren M, Wang B, Apanasets O. Biochim Biophys Acta. Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Guo Z, Deshpande R, Paull TT. ATM activation in the presence of oxidative stress. Cell Cycle. Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Tripathi DN, Chowdhury R, Trudel LJ, Tee AR, Slack RS, Walker CL, Wogan GN. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Zhang J, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, Dere R, Tait-Mulder J, Lee JH, Paull TT, et al. ATM functions at the peroxisome to induce pexophagy in response to ROS. Kamsler A, Daily D, Hochman A, Stern N, Shiloh Y, Rotman G, Barzilai A. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Reichenbach J, Schubert R, Schindler D, Muller K, Bohles H, Zielen S. Elevated oxidative stress in patients with ataxia telangiectasia. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Callahan MK, Chaillot D, Jacquin C, Clark PR, Menoret A. Differential acquisition of antigenic peptides by Hsp70 and Hsc70 under oxidative conditions. Lee JJ, Ishihara K, Notomi S, Efstathiou NE, Ueta T, Maidana D, Chen X, Iesato Y, Caligiana A, Vavvas DG. Lysosome-associated membrane protein-2 deficiency increases the risk of reactive oxygen species-induced ferroptosis in retinal pigment epithelial cells. Biochem Biophys Res Commun. Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Suzuki T, Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. Suzuki T, Muramatsu A, Saito R, Iso T, Shibata T, Kuwata K, Kawaguchi SI, Iwawaki T, Adachi S, Suda H, et al. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. Friling RS, Bergelson S, Daniel V. Two adjacent APlike binding sites form the electrophile-responsive element of the murine glutathione S-transferase Ya subunit gene. Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. Telakowski-Hopkins CA, King RG, Pickett CB. Glutathione S-transferase Ya subunit gene: identification of regulatory elements required for basal level and inducible expression. Pajares M, Jimenez-Moreno N, Garcia-Yague AJ, Escoll M, de Ceballos ML, Van Leuven F, Rabano A, Yamamoto M, Rojo AI, Cuadrado A. Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. Kaspar JW, Niture SK, Jaiswal AK. Nrf 2:INrf2 Keap1 signaling in oxidative stress. Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Kageyama S, Gudmundsson SR, Sou YS, Ichimura Y, Tamura N, Kazuno S, Ueno T, Miura Y, Noshiro D, Abe M, et al. Kumar RR, Narasimhan M, Shanmugam G, Hong J, Devarajan A, Palaniappan S, Zhang J, Halade GV, Darley-Usmar VM, Hoidal JR, et al. Abrogation of Nrf2 impairs antioxidant signaling and promotes atrial hypertrophy in response to high-intensity exercise stress. J Transl Med. Georgakopoulos ND, Frison M, Alvarez MS, Bertrand H, Wells G, Campanella M. Reversible Keap1 inhibitors are preferential pharmacological tools to modulate cellular mitophagy. Sci Rep. Murata H, Takamatsu H, Liu S, Kataoka K, Huh NH, Sakaguchi M. NRF2 regulates PINK1 expression under oxidative stress conditions. PLoS ONE. Yamada T, Murata D, Adachi Y, Itoh K, Kameoka S, Igarashi A, Kato T, Araki Y, Huganir RL, Dawson TM, et al. Mitochondrial stasis reveals pmediated ubiquitination in parkin-independent mitophagy and mitigates nonalcoholic fatty liver disease. Cell Metab. Bialik S, Dasari SK, Kimchi A. Autophagy-dependent cell death—where, how and why a cell eats itself to death. Google Scholar. Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. Ha S, Ryu HY, Chung KM, Baek SH, Kim EK, Yu SW. Regulation of autophagic cell death by glycogen synthase kinase-3beta in adult hippocampal neural stem cells following insulin withdrawal. Mol Brain. Law BYK, Michelangeli F, Qu YQ, Xu SW, Han Y, Mok SWF, Dias I, Javed MU, Chan WK, Xue WW, et al. Quigley HA, Broman AT. The number of people with glaucoma worldwide in and Br J Ophthalmol. Nettesheim A, Dixon A, Shim MS, Coyne A, Walsh M, Liton PB. Autophagy in the aging and experimental ocular hypertensive mouse model. Invest Ophthalmol Vis Sci. Lin WJ, Kuang HY. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Amankwa CE, Gondi SR, Dibas A, Weston C, Funk A, Nguyen T, Nguyen KT, Ellis DZ, Acharya S. Novel thiol containing hybrid antioxidant-nitric oxide donor small molecules for treatment of glaucoma. Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. Shim MS, Nettesheim A, Dixon A, Liton PB. Zuo L, Khan RS, Lee V, Dine K, Wu W, Shindler KS. SIRT1 promotes RGC survival and delays loss of function following optic nerve crush. Kang LH, Zhang S, Jiang S, Hu N. Activation of autophagy in the retina after optic nerve crush injury in rats. Int J Ophthalmol. Zhan Z, Wu Y, Liu Z, Quan Y, Li D, Huang Y, Yang S, Wu K, Huang L, Yu M. Reduced dendritic spines in the visual cortex contralateral to the optic nerve crush eye in adult mice. Kim SH, Munemasa Y, Kwong JM, Ahn JH, Mareninov S, Gordon LK, Caprioli J, Piri N. Activation of autophagy in retinal ganglion cells. J Neurosci Res. Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tonges L, Stadelmann C, Bruck W, Bahr M, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Rodriguez-Muela N, Germain F, Marino G, Fitze PS, Boya P. Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Porter K, Hirt J, Stamer WD, Liton PB. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Kitaoka Y, Sase K, Tsukahara C, Fujita N, Tokuda N, Kogo J, Takagi H. Axonal protection by a small molecule SIRT1 activator, SRT, with alteration of autophagy in TNF-induced optic nerve degeneration. Jpn J Ophthalmol. Yazdankhah M, Ghosh S, Shang P, Stepicheva N, Hose S, Liu H, Chamling X, Tian S, Sullivan MLG, Calderon MJ, et al. BNIP3L-mediated mitophagy is required for mitochondrial remodeling during the differentiation of optic nerve oligodendrocytes. Beckers A, Vanhunsel S, Van Dyck A, Bergmans S, Masin L, Moons L. Injury-induced autophagy delays axonal regeneration after optic nerve damage in adult zebrafish. Rodriguez-Muela N, Boya P. Axonal damage, autophagy and neuronal survival. Ying H, Yue BY. Optineurin: the autophagy connection. Exp Eye Res. Zhang S, Shao Z, Liu X, Hou M, Cheng F, Lei D, Yuan H. The E50K optineurin mutation impacts autophagy-mediated degradation of TDP and leads to RGC apoptosis in vivo and in vitro. Cell Death Discov. Losiewicz MK, Elghazi L, Fingar DC, Rajala RVS, Lentz SI, Fort PE, Abcouwer SF, Gardner TW. mTORC1 and mTORC2 expression in inner retinal neurons and glial cells. Russo R, Berliocchi L, Adornetto A, Amantea D, Nucci C, Tassorelli C, Morrone LA, Bagetta G, Corasaniti MT. In search of new targets for retinal neuroprotection: is there a role for autophagy? Curr Opin Pharmacol. Russo R, Varano GP, Adornetto A, Nazio F, Tettamanti G, Girardello R, Cianfanelli V, Cavaliere F, Morrone LA, Corasaniti MT, et al. Bell K, Rosignol I, Sierra-Filardi E, Rodriguez-Muela N, Schmelter C, Cecconi F, Grus F, Boya P. Age related retinal Ganglion cell susceptibility in context of autophagy deficiency. Kauppinen A. Introduction to the multi-author review on macular degeneration. Cell Mol Life Sci. Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY. Age-related macular degeneration. Nat Rev Dis Primers. Chan CM, Huang DY, Sekar P, Hsu SH, Lin WW. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J Biomed Sci. Zhang ZY, Bao XL, Cong YY, Fan B, Li GY. Autophagy in age-related macular degeneration: a regulatory mechanism of oxidative stress. Wang S, Ji LY, Li L, Li JM. Oxidative stress, autophagy and pyroptosis in the neovascularization of oxygeninduced retinopathy in mice. Mol Med Rep. Song C, Mitter SK, Qi X, Beli E, Rao HV, Ding J, Ip CS, Gu H, Akin D, Dunn WA Jr, et al. Blasiak J, Szczepanska J, Fila M, Pawlowska E, Kaarniranta K. Potential of telomerase in age-related macular degeneration-involvement of Senescence, DNA damage response and autophagy and a key role of PGC-1alpha. Yang X, Pan X, Zhao X, Luo J, Xu M, Bai D, Hu Y, Liu X, Yu Q, Gao D. Autophagy and age-related eye diseases. Biomed Res Int. George SM, Lu F, Rao M, Leach LL, Gross JM. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog Retin Eye Res. Kaarniranta K, Tokarz P, Koskela A, Paterno J, Blasiak J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol Toxicol. Liu J, Copland DA, Theodoropoulou S, Chiu HA, Barba MD, Mak KW, Mack M, Nicholson LB, Dick AD. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. Rodriguez-Muela N, Koga H, Garcia-Ledo L, de la Villa P, de la Rosa EJ, Cuervo AM, Boya P. Balance between autophagic pathways preserves retinal homeostasis. Aging Cell. Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W Jr, Ding J, et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Szatmari-Toth M, Kristof E, Vereb Z, Akhtar S, Facsko A, Fesus L, Kauppinen A, Kaarniranta K, Petrovski G. Clearance of autophagy-associated dying retinal pigment epithelial cells—a possible source for inflammation in age-related macular degeneration. Szatmari-Toth M, Ilmarinen T, Mikhailova A, Skottman H, Kauppinen A, Kaarniranta K, Kristof E, Lytvynchuk L, Vereb Z, Fesus L, et al. Human embryonic stem cell-derived retinal pigment epithelium-role in dead cell clearance and inflammation. Sheu SJ, Chen JL, Bee YS, Lin SH, Shu CW. ERBB2-modulated ATG4B and autophagic cell death in human ARPE19 during oxidative stress. Chang KC, Snow A, LaBarbera DV, Petrash JM. Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells. Chem Biol Interact. Yao J, Tao ZF, Li CP, Li XM, Cao GF, Jiang Q, Yan B. Regulation of autophagy by high glucose in human retinal pigment epithelium. Piano I, Novelli E, Della Santina L, Strettoi E, Cervetto L, Gargini C. Involvement of autophagic pathway in the progression of retinal degeneration in a mouse model of diabetes. Front Cell Neurosci. Coucha M, Elshaer SL, Eldahshan WS, Mysona BA, El-Remessy AB. Molecular mechanisms of diabetic retinopathy: potential therapeutic targets. Middle East Afr J Ophthalmol. Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species ROS and diabetic complications. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Rosa MD, Distefano G, Gagliano C, Rusciano D, Malaguarnera L. Autophagy in diabetic retinopathy. Curr Neuropharmacol. Fu D, Wu M, Zhang J, Du M, Yang S, Hammad SM, Wilson K, Chen J, Lyons TJ. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Aihara M. Prostanoid receptor agonists for glaucoma treatment. Skov AG, Rives AS, Freiberg J, Virgili G, Azuara-Blanco A, Kolko M: Comparative efficacy and safety of preserved versus preservative-free beta-blockers in patients with glaucoma or ocular hypertension: a systematic review. Acta Ophthalmol Nocentini A, Supuran CT. Adrenergic agonists and antagonists as antiglaucoma agents: a literature and patent review — Expert Opin Ther Pat. Jansook P, Hnin HM, Loftsson T, Stefansson E. Cyclodextrin-based formulation of carbonic anhydrase inhibitors for ocular delivery—a review. Int J Pharm. Al-Humimat G, Marashdeh I, Daradkeh D, Kooner K. Investigational Rho kinase inhibitors for the treatment of glaucoma. J Exp Pharmacol. Faiq MA, Wollstein G, Schuman JS, Chan KC. Cholinergic nervous system and glaucoma: from basic science to clinical applications. Toteberg-Harms M, Meier-Gibbons F. Is laser trabeculoplasty the new star in glaucoma treatment? Curr Opin Ophthalmol. Wolters JEJ, van Mechelen RJS, Al Majidi R, Pinchuk L, Webers CAB, Beckers HJM, Gorgels T. History, presence, and future of mitomycin C in glaucoma filtration surgery. Riva I, Roberti G, Katsanos A, Oddone F, Quaranta L. A Review of the ahmed glaucoma valve implant and comparison with other surgical operations. Adv Ther. Kan JT, Betzler BK, Lim SY, Ang BCH. Anterior segment imaging in minimally invasive glaucoma surgery—a systematic review. Acta Ophthalmol. Article PubMed Google Scholar. Chang KC, Sun C, Cameron EG, Madaan A, Wu S, Xia X, Zhang X, Tenerelli K, Nahmou M, Knasel CM, et al. Opposing effects of growth and differentiation factors in cell-fate specification. Curr Biol. Zhang X, Tenerelli K, Wu S, Xia X, Yokota S, Sun C, Galvao J, Venugopalan P, Li C, Madaan A, et al. Cell transplantation of retinal ganglion cells derived from hESCs. Restor Neurol Neurosci. Fligor CM, Langer KB, Sridhar A, Ren Y, Shields PK, Edler MC, Ohlemacher SK, Sluch VM, Zack DJ, Zhang C, et al. Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Sluch VM, Chamling X, Liu MM, Berlinicke CA, Cheng J, Mitchell KL, Welsbie DS, Zack DJ. Enhanced stem cell differentiation and immunopurification of genome engineered human retinal ganglion cells. Stem Cells Transl Med. Luo Z, Xian B, Li K, Li K, Yang R, Chen M, Xu C, Tang M, Rong H, Hu D, et al. scaffolds facilitate epiretinal transplantation of hiPSC-derived retinal neurons in nonhuman primates. Acta Biomater. Miltner AM, La Torre A. Retinal ganglion cell replacement: current status and challenges ahead. Dev Dyn. Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. Chang KC, Bian M, Xia X, Madaan A, Sun C, Wang Q, Li L, Nahmou M, Noro T, Yokota S, et al. Posttranslational modification of Sox11 regulates RGC survival and axon regeneration. Xie L, Yin Y, Benowitz L. Chemokine CCL5 promotes robust optic nerve regeneration and mediates many of the effects of CNTF gene therapy. Patel AK, Broyer RM, Lee CD, Lu T, Louie MJ, La Torre A, Al-Ali H, Vu MT, Mitchell KL, Wahlin KJ, et al. Inhibition of GCK-IV kinases dissociates cell death and axon regeneration in CNS neurons. Williams PR, Benowitz LI, Goldberg JL, He Z. Axon regeneration in the mammalian optic nerve. Annu Rev Vis Sci. Matloob S, Fan JC, Danesh-Meyer HV. Multifocal malignant optic glioma of adulthood presenting as acute anterior optic neuropathy. J Clin Neurosci. Tooley AA, Rasool N, Campbell A, Kazim M. Acute angle plication of optic nerve glioma as a mechanism of rapidly progressive visual loss. Pan Y, Hysinger JD, Barron T, Schindler NF, Cobb O, Guo X, Yalcin B, Anastasaki C, Mulinyawe SB, Ponnuswami A, et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Brown EE, DeWeerd AJ, Ildefonso CJ, Lewin AS, Ash JD. Mitochondrial oxidative stress in the retinal pigment epithelium RPE led to metabolic dysfunction in both the RPE and retinal photoreceptors. Skrzypczak T, Jany A, Bugajska-Abramek E, Boguslawska J, Kowal-Lange A. A comparative study of ranibizumab and aflibercept for neovascular age-related macular degeneration: month outcomes of Polish therapeutic program in non-tertiary institution. Banaee T, Alwan S, Kellogg C, Kornblau I, El-Annan J. PRN treatment of neovascular AMD with cycles of three monthly injections. J Ophthalmic Vis Res. Parravano M, Costanzo E, Scondotto G, Trifiro G, Virgili G. Anti-VEGF and other novel therapies for neovascular age-related macular degeneration: an update. Chichan H, Maus M, Heindl LM. Subthreshold nanosecond laser, from trials to real-life clinical practice: a cohort study. Clin Ophthalmol. Gao Y, Yu T, Zhang Y, Dang G. Anti-VEGF monotherapy versus photodynamic therapy and anti-VEGF combination treatment for neovascular age-related macular degeneration: a meta-analysis. Bikbov MM, Orenburkina OI, Babushkin AE, Burkhanov YK. Use of macular lenses in patients with age-related macular degeneration. Vestn Oftalmol. Arrigo A, Bandello F. Molecular features of classic retinal drugs, retinal therapeutic targets and emerging treatments. Berrocal MH, Acaba-Berrocal L. Early pars plana vitrectomy for proliferative diabetic retinopathy: update and review of current literature. Chang KC, Petrash JM. Aldo-Keto reductases: multifunctional proteins as therapeutic targets in diabetes and inflammatory disease. Sonowal H, Ramana KV. Development of aldose reductase inhibitors for the treatment of inflammatory disorders and cancer: current drug design strategies and future directions. Curr Med Chem. Suzen S, Buyukbingol E. Recent studies of aldose reductase enzyme inhibition for diabetic complications. Gabbay KH. Aldose reductase inhibition in the treatment of diabetic neuropathy: where are we in ? Curr Diab Rep. Ishikawa M, Takaseki S, Yoshitomi T, Covey DF, Zorumski CF, Izumi Y. Sbardella D, Tundo GR, Coletta M, Manni G, Oddone F. Dexamethasone downregulates autophagy through accelerated turn-over of the Ulk-1 complex in a trabecular meshwork cells strain: insights on steroid-induced glaucoma pathogenesis. Hamano T, Shirafuji N, Yen SH, Yoshida H, Kanaan NM, Hayashi K, Ikawa M, Yamamura O, Fujita Y, Kuriyama M, et al. Rho-kinase ROCK inhibitors reduce oligomeric tau protein. Neurobiol Aging. Kitaoka Y, Sase K, Tsukahara C, Kojima K, Shiono A, Kogo J, Tokuda N, Takagi H. Axonal protection by ripasudil, a rho kinase inhibitor, via modulating autophagy in TNF-induced optic nerve degeneration. Zhang J, Bai Y, Huang L, Qi Y, Zhang Q, Li S, Wu Y, Li X. Protective effect of autophagy on human retinal pigment epithelial cells against lipofuscin fluorophore A2E: implications for age-related macular degeneration. Ran Z, Zhang Y, Wen X, Ma J. Su W, Li Z, Jia Y, Zhuo Y. Rapamycin is neuroprotective in a rat chronic hypertensive glaucoma model. Dai Y, Zheng K, Clark J, Swerdlow RH, Pulst SM, Sutton JP, Shinobu LA, Simon DK. Rapamycin drives selection against a pathogenic heteroplasmic mitochondrial DNA mutation. Hum Mol Genet. Cinik R, Yuksel N, Pirhan D, Aslan MS, Subasi C, Karaoz E. The effect of everolimus on scar formation in glaucoma filtering surgery in a rabbit model. Curr Eye Res. Matsuki M, Adachi Y, Ozawa Y, Kimura T, Hoshi T, Okamoto K, Tohyama O, Mitsuhashi K, Yamaguchi A, Matsui J, et al. Targeting of tumor growth and angiogenesis underlies the enhanced antitumor activity of lenvatinib in combination with everolimus. Cancer Sci. Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. Uchida J, Iwai T, Nakatani T. Introduction of everolimus in kidney transplant recipients at a late posttransplant stage. World J Transpl. Touhami S, Arzouk N, Darugar A, Heron E, Clarencon F, Bodaghi B, LeHoang P, Barrou B, Touitou V. Everolimus-induced posterior reversible encephalopathy syndrome and bilateral optic neuropathy after kidney transplantation. Canovai E, Cassiman C, Ceulemans LJ, Demaerel P, Sainz-Barriga M, Jochmans I, Monbaliu D, Pirenne J, Vanuytsel T. Tacrolimus-induced optic neuropathy after multivisceral transplantation. Transpl Direct. Liegl R, Koenig S, Siedlecki J, Haritoglou C, Kampik A, Kernt M. Temsirolimus inhibits proliferation and migration in retinal pigment epithelial and endothelial cells via mTOR inhibition and decreases VEGF and PDGF expression. Jacot JL, Sherris D. J Ophthalmol. Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. Brown EE, Ball JD, Chen Z, Khurshid GS, Prosperi M, Ash JD. The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Lin HC, Stein JD, Nan B, Childers D, Newman-Casey PA, Thompson DA, Richards JE. Association of geroprotective effects of metformin and risk of open-angle glaucoma in persons with diabetes mellitus. JAMA Ophthalmol. Kim YS, Kim M, Choi MY, Lee DH, Roh GS, Kim HJ, Kang SS, Cho GJ, Kim SJ, Yoo JM, et al. Metformin protects against retinal cell death in diabetic mice. Motoi Y, Shimada K, Ishiguro K, Hattori N. Lithium and autophagy. ACS Chem Neurosci. Sun XB, Lu HE, Chen Y, Fan XH, Tong B. Zeilbeck LF, Muller B, Knobloch V, Tamm ER, Ohlmann A. Differential angiogenic properties of lithium chloride in vitro and in vivo. Ruiz-Pesini E, Emperador S, Lopez-Gallardo E, Hernandez-Ainsa C, Montoya J. Increasing mtDNA levels as therapy for mitochondrial optic neuropathies. Drug Discov Today. Fauzi YR, Nakahata S, Chilmi S, Ichikawa T, Nueangphuet P, Yamaguchi R, Nakamura T, Shimoda K, Morishita K. Zhang ML, Zhao GL, Hou Y, Zhong SM, Xu LJ, Li F, Niu WR, Yuan F, Yang XL, Wang Z, et al. Rac1 conditional deletion attenuates retinal ganglion cell apoptosis by accelerating autophagic flux in a mouse model of chronic ocular hypertension. Kan E, Yakar K, Demirag MD, Gok M. Macular ganglion cell-inner plexiform layer thickness for detection of early retinal toxicity of hydroxychloroquine. Int Ophthalmol. Reichel C, Berlin A, Radun V, Tarau IS, Hillenkamp J, Kleefeldt N, Sloan KR, Ach T. Transl Vis Sci Technol. Singh DK, Muhieddine L, Einstadter D, Ballou S. Incidence of blindness in a population of rheumatic patients treated with hydroxychloroquine. Rheumatol Adv Pract. Amato R, Catalani E, Dal Monte M, Cammalleri M, Di Renzo I, Perrotta C, Cervia D, Casini G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol Res. Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Fujita Y, Inagaki N. Metformin: clinical topics and new mechanisms of action. Diabetol Int. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Meng D, Frank AR, Jewell JL. mTOR signaling in stem and progenitor cells. Download references. The authors would like to acknowledge the published work that were cited for this review and those that were not cited because of space and our own limitations. The work was supported by the NIH CORE Grant P30 EY to the Department of Ophthalmology University of Pittsburgh, the Eye and Ear Foundation of Pittsburgh, and from an unrestricted grant from Research to Prevent Blindness, New York, NY, Ministry of Science and Technology MOST BMY3 and BMY3 and the National Sun Yat-sen University-KCGMH Joint Research Project and Department of Ophthalmology and Neurobiology, Louis J. Fox Center for Vision Restoration, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA. Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan. Department of Biomedical Science and Environmental Biology, PhD Program in Life Science, College of Life Science, Kaohsiung Medical University, Kaohsiung, Taiwan. Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan. Center for Cancer Research, Kaohsiung Medical University, Kaohsiung, Taiwan. Institute of BioPharmaceutical Sciences, National Sun Yat-Sen University, No. Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan. Institute of Clinical Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan. Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan. Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan. You can also search for this author in PubMed Google Scholar. KCC, PFL, YCL, YJC and CWS wrote the manuscript. CHC generated the figures and table. All authors read and approved the final manuscript. Correspondence to Chih-Wen Shu. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Chang, KC. et al. The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases. Cell Biosci 12 , 1 Download citation. Received : 25 November Accepted : 19 December Published : 03 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Review Open access Published: 03 January The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases Kun-Che Chang ORCID: orcid. Abstract Oxidative stress is mainly caused by intracellular reactive oxygen species ROS production, which is highly associated with normal physiological homeostasis and the pathogenesis of diseases, particularly ocular diseases. Background Christian de Duve, a Nobel Prize winner in , observed cellular autophagic structures by electron microscopy sixty years ago due to the discovery of peroxisomes and lysosomes [ 1 , 2 ]. Macroautophagy Since macroautophagy is the most common form of autophagy, autophagy usually means macroautophagy. CMA CMA is a chaperone HSC70 -dependent degradation pathway. Microautophagy Microautophagy was defined as micro portion of lysosomal membrane to engulf autophagic cargos, including proteins and organelles, in cells [ 17 ]. Autophagy-related proteins There are more than 40 Atg proteins involved in macroautophagy signaling in yeast cells. |
| Oxidative Stress and Autophagy | IntechOpen | In conclusion, oxidative stress and autophagy regulate each other, with autophagy regulating redox homeostasis through the Nrf2 and SESNs antioxidant pathways. COPD is a respiratory disease caused by direct and long-term exposure to toxic particulates or gases. It triggers airway or alveolar abnormalities, leading to symptoms of chronic bronchitis and emphysema, which usually manifest as persistent respiratory symptoms and airflow restriction Rodriguez-Roisin et al. CS is a major risk factor for COPD, and thus the mortality rate of COPD among smokers is higher than that of non-smokers Vestbo et al. Notably, CS is a complex mixture containing 4, chemical components, including carbon monoxide, heavy metals, aldehydes, aromatic hydrocarbons, free radicals, and other oxidizing compounds Church and Pryor, Studies have shown that although e-cigarettes produce fewer toxic substances than traditional cigarettes, they also contain nicotine, also making them potentially harmful to the lungs Zhang et al. Our previous studies found that exposure to CS significantly reduced the expression levels of protein tyrosine phosphatase-like Adomain containing 2 PTPLAD2 and ubiquitin specific peptidase 49 USP49 in BEAS-2B cells, suggesting that these genes might play a key role in CSE-induced COPD Zhang et al. The primary targets of inhaled CS are airway and alveolar epithelial cells Racanelli et al. Exposure to CS, uncontrolled chronic inflammation and oxidative stress are the main drivers of the pathogenesis of COPD in airway epithelial cells, and are involved in many forms of regulated cell death i. Smoking is a major cause of systemic oxidative stress, excessive inflammation, and emphysema. Patients with COPD are continuously exposed to high levels of oxidative stress and lung inflammation, which can lead to airway obstruction and destruction of the lung parenchyma Tan et al. The large oxidative burden, caused by mitochondrial dysfunction, has been confirmed to be the principal cause of abnormal response and refractory inflammation due to exposure to CS Jiang et al. In addition, the free radical theory states that oxidative stress is the primary driving factor leading to cellular aging Radak et al. Autophagy is a process of homeostatic degradation of organelles or proteins involved in oxidative stress damage, and also plays a role in regulating inflammation by regulating the development and survival of inflammatory cells Qian et al. A number of studies have confirmed the importance of oxidative stress for inducing lung disorders. These studies have determined the existence of free radical biomarkers that induce lung inflammation and autoimmune responses and damage in patients with COPD. The principal sources of ROS and RNS in the lungs are the environment and cells Yao and Rahman, Injury and exposure to triggers stimulates the production of endogenous oxidation products by epithelial cells, endothelial cells, airway cells, and alveolar macrophages, and also recruits inflammatory cells to the lungs, generating additional oxidative stress Rogers and Cismowski, ROS and RNS are also known to be produced by various inflammatory and structural cells in the airway. One of the characteristics of COPD is its inflammatory immune response, which is characterized by the recruitment and activation of epithelial cells and macrophages, neutrophils, monocytes, and lymphocytes. In particular, once inflammatory cells are recruited into the airway, they are activated, producing ROS Yao and Rahman, Some researchers have found that CS participates in the progression of COPD by inducing the M1 and M2 polarization of macrophages Feng et al. Oxidases, including nicotinamide adenine dinucleotide phosphate NADPH oxidase NOXs , are the primary source of oxidative stress in the lungs. Studies have found that NOXs produce a large amount of oxidative agents that protect the lungs Al Ghouleh et al. Lung hypoxia, ischemic injury, and airway inflammation irreversibly convert peroxisomal xanthine dehydrogenase into xanthine oxidase, which is the principal source of the production of superoxide and is also involved in COPD process Rogers and Cismowski In addition, the pulmonary endothelium also upregulates the production of NO by increasing the NOS activity. The balance of autophagy plays an important role in maintaining the dynamics of the intracellular environment. Nonetheless, COPD can cause cellular damage severe enough to trigger autophagy in lung cells Dan Dunn et al. As CS inhalation inactivates proteases necessary for protecting the lungs, the development and progression of COPD have been closely related to the oxidation of essential proteins and lipids in the airway epithelium and sputum and the decrease in the levels of antioxidants, such as glutathione and superoxide dismutase Drost et al. The pathogenesis of COPD has been associated with an excessive increase in autophagy and mitophagy, which lead to programmed cell death of epithelial cells and emphysema Chen et al. Mutations in the ATG16L1 autophagic gene constitute a major risk factor for susceptibility to COPD. Autophagy is also increased in the lung epithelium of patients with mutations in emphysema genes, such as 1-antitrypsin deficiency 1-AT ; however, its etiology is independent of smoke or particulate inhalation Chen et al. CS has been shown to cause abnormal autophagy and mitophagy through apoptosis, leading to programmed cell death in bronchial cells Chen et al. Studies have shown a significant increase in the levels of autophagic proteins in the lung tissues of patients with COPD at different stages of disease Chen et al. In contrast, inhibiting autophagy by silencing LC3B protected epithelial cells from CSE-induced apoptosis Chen et al. Moreover, the activity of histone deacetylase HDAC was reduced in the lungs of patients with COPD, whereas the expression of the LC3-II autophagosome formation marker and that of other autophagy-related proteins, including ATG4b, ATG5, ATG12, and ATG7, was significantly increased and associated with the increased activation of caspase-3 Chen et al. Electron microscopy analysis of lung tissues of patients with COPD showed that the production of autophagosomes was increased in their lungs compared with to that in the lungs of the control group. The same phenomenon was also observed in animal experiments. The lungs of CS-exposed mice exhibited increased formation of autophagosomes under electron microscopy observations, as well as increased expression of LC3B-II Chen et al. The initial suggestion of the importance of autophagy in the progression of COPD came from studies on upstream regulators of autophagy, such as toll-like receptor 4 TLR4 and early growth response-1 EGR In these studies, inhibition of autophagy limited in vivo inflammation, cell dysfunction, and apoptosis observed with chronic exposure to CS Chen et al. Overexpression of exogenous superoxide dismutase SOD reduced the levels of expression of early growth response 1 Egr-1 gene and protein, a transcription factor essential for hypoxia-related autophagy in the lungs Nozik-Grayck et al. Studies have shown that CSE reduces the activity of HDAC in lung epithelial cells, thereby increasing the binding of Egr-1 and the E2F transcription factor to the LC3B promoter, thus increasing the expression of LC3B Chen et al. Consequently, the CS-mediated reduction in the activity of HDAC leads to the transcriptional activation of Egr-1 and E2F-4, thereby inducing autophagic death Chen et al. These results suggested that regulating the autophagic pathway might be beneficial in COPD interventions Yao and Rahman, Knockdown of LC3b was reported to inhibit the activation and apoptosis of caspase-3 and improve cell viability in bronchial epithelial cells exposed to CSE, which was consistent with the view that autophagy is harmful Chen et al. Furthermore, the increased expression of the early growth response protein 1 EGR-1 transcription factor was shown to be necessary for increasing the levels of LC3B and ATG4B Egrdeficient mice exhibited a decrease in the levels of LC3B-II and ATG4B after exposure to CS, thereby mitigating the development of emphysema Chen et al. Studies in mice exposed to CS for 16 weeks showed that CS induced autophagy in neutrophils through the activation of platelet-activating factor receptor PAFR. Conversely, blockade of PAFR with rupatadine reduced the autophagic death of neutrophils, thereby reducing emphysema Lv et al. The activity of LC3B is regulated by a variety of membrane-associated and cytoplasmic factors. Studies have found that LC3B is bound to the Fas complex, a component of the DISC, in a manner dependent on the caveolin-1 caveolae-scaffolding protein. Exposure to CS has been shown to result in the rapid dissociation of LC3B from the Fas complex, consistent with the activation of the extrinsic apoptotic pathway Nakahira et al. Accordingly, mutations in the caveolin-1 binding motif of LC3B have been reported to attenuate the proapoptotic effect resulting from the expression of LC3 Chen et al. Deletion of the LC3B autophagic protein inhibited CS-induced airspace enlargement in vivo Chen et al. Our previous studies showed the protective effects of IP 3 R against damage in extracted smoke solution ESS -treated HBE cells, which was achieved by reducing oxidative stress. Some researchers have observed that CS can induce the deposition of proteases, thereby driving the accumulation of ubiquitinated proteins aggregates in epithelial cells and exacerbating chronic inflammation van Rijt et al. Oxidative stress-induced increases in histone deacetylase-6 have been associated with autophagic degradation and shortening of bronchial cilia, suggesting mucociliary dysfunction Harris and Rubinsztein. Fujii et al. found that CS activated autophagy in human bronchial epithelial cells isolated from patients with COPD, leading to increased cell senescence and accumulation of the p62 autophagic adaptor protein and several ubiquitinated proteins Fujii et al. Inhibition of autophagy was shown to further increase the levels of p62 and ubiquitinated proteins Fujii et al. Researchers have speculated that the accumulation of p62 and ubiquitinated proteins observed in the lung tissues of patients with severe COPD-emphysema suggests an insufficient autophagic clearance is involved in the pathogenesis of COPD Tran et al. Racanelli et al. found that the CS-induced excessive autophagy and mitophagy led to bronchial cell apoptosis and necroptosis, respectively, thereby providing a possible mechanism for the development of emphysema Racanelli et al. The activation of mitochondrial selective autophagy, namely the connection between mitophagy and other regulated forms of cell death, such as apoptosis and necroptosis, is a driving factor of the COPD phenotype and underscores its importance in normal lung homeostasis and pathogenesis Hou et al. CS-induced mitochondrial dysfunction and loss of mitochondrial phagocytosis have also been reported to induce cellular senescence and progression of COPD. Recent studies have shown that oxidative stress can accelerate aging by depleting stem cells, thereby causing accumulation of dysfunctional mitochondria, and decreasing autophagy, all of which generate additional oxidative stress Mercado et al. Numerous studies have shown that CSE causes accumulation of damaged mitochondria with severe mitochondrial damage through mitophagy Mizumura et al. Likewise, CS-induced endogenous ROS are known to stimulate the production of mitochondrial fragments in primary human bronchial epithelial cells. These mitochondrial fragments produce additional ROS, accelerating cellular aging Hoffmann et al. Induction of mitochondrial autophagy reduces the production of ROS by removing damaged mitochondria, conferring a significant protective effect on human bronchial epithelial cells Ito et al. As such, mitochondrial phagocytosis might downregulate excessive inflammation and serve as a protective mechanism in patients with COPD Yao et al. Inhibiting the activation of mitochondrial phagocytosis by inhibiting either the PINK or PRKN signaling pathways led to the increased production of ROS and activation of inflammasome in small airway epithelial cells of patients with COPD Ito et al. In general, CSE has been confirmed to induce mitochondrial dysfunction, damage mitochondrial phagocytosis, and cause accumulation of damaged mitochondrial DNA Ahmad et al. Parkin is a key regulator of mitochondrial phagocytosis and has been shown to be downregulated in tissues of patients with COPD Ito et al. Therefore, reversing dysfunctional mitochondrial phagocytosis should be the first choice for the treatment of COPD. In vitro experiments, overexpression of Parkin in epithelial cells resulted in inhibition of mitochondrial production of ROS, whereas CS extracts caused cell senescence Araya et al. In vivo and in vitro studies showed that the increased levels of Parkin enhance mitochondrial phagocytosis and disrupt the progression of COPD Araya et al. Autophagy is one of the most important biological responses for regulating the levels of ROS and oxidative stress in cells Mizushima, through the clearance and degradation of damaged mitotic and oxidized proteins Yao and Rahman, For example, sirtuin 1 SIRT1 , a type III histone deacetylase, was reported to positively regulate mitochondrial phagocytosis by upregulating the expression of the peroxisome proliferator-activated receptor-γ coactivator 1α PGC-1α ; the expression of PGC-1α decreased in the lungs of patients with moderate and severe COPD El-Khamisy et al. This relationship between mitochondrial phagocytosis and necroptosis is essential in the progression and outcomes of patients with COPD. The molecular mechanism and molecular entities involved in autophagy during oxidative stress in COPD are summarized in Figure 3 and Figure 4 , respectively. FIGURE 3. Molecular mechanisms of autophagy during oxidative stress in COPD. CS induces activation of oxidative stress and production of reactive oxygen species ROS. The generation of ROS then triggers the formation of apoptosis and senescence, and degradation of autophagy and mitophagy, leading to emphysema and shortening of bronchial cilia, and ultimately induces COPD. FIGURE 4. List of molecular entities involved in autophagy during oxidative stress in COPD. ROS induced by CS involve different molecular entities in apoptosis, senescence, autophagy and mitophagy in the pathogenesis of chronic obstructive pulmonary disease COPD. An increasing body of evidence has indicated that autophagy in a variety of cell types plays a major role in the pathogenesis of COPD. Both excessive and insufficient autophagy drives the inflammation, cell death, and cell dysfunction that are observed in COPD. Treatment options for COPD remain rather limited, and hence the potential of targeted autophagy as a treatment for COPD warrants further investigation Racanelli et al. Currently, only a few modulators of autophagy have been evaluated for clinical use, including rapamycin an activator of autophagy and chloroquine or hydroxychloroquine inhibitors of autophagy. Likewise, strategies for the treatment of COPD might involve drugs that target autophagic proteins or modulate the selective clearance and turnover of autophagy Nakahira et al. The use of the rapamycin mTOR inhibitor showed that although increasing autophagy to reducealveolar inflammation after exposure to CS might be beneficial, rapamycin increased autophagy and the number of apoptotic and inflammatory cells in control mice after indoor air exposure. These findings underscored the complexity of targeted autophagy as a treatment modality for COPD Yoshida et al. To date, the number of clinical trials evaluating autophagy modulation therapy in patients with COPD remains insufficient. This might be due to the consideration that increased autophagy is always beneficial for patients, and to the lack of a reliable method to study the effects of autophagy in patients. Another reason might be that autophagy is a controversial process, as insufficient autophagy results in aging, whereas excessive autophagy results in cell death. The discovery of therapeutic drugs that modulate autophagy in COPD is still in the early stages, and many clinically effective drugs are being repositioned to promote autophagy in COPD in vivo and in vitro models Tan et al. However, further research is needed to critically evaluate the role of these drugs in the treatment of abnormal autophagy in COPD. A number of natural and synthetic compounds have been shown to counteract CS-induced oxidative stress. These compounds have antioxidant activity and can be used to ameliorate chronic inflammatory and injurious responses in COPD. For example, 3, 4, 5-trihydroxyhexanostyrene resveratrol , a plant polyphenol, has been shown to have both anti-inflammatory and antioxidant functions, inhibit CS-induced autophagy and improve the prognosis of COPD Hwang et al. Spermidine is a natural polyamine that restores autophagy activity and reduces oxidative stress de Cabo et al. Rapamycin can reverse defective antioxidant responses or inhibit the mTOR pathway to reduce oxidative stress damage, which can prevent aging and chronic inflammation Mercado et al. N-acetylcysteine has anti-inflammatory and antioxidant effects Vanella et al. These antioxidants bring new directions for the treatment of chronic inflammatory lung diseases such as COPD. Autophagy is a generalized response to oxidative stress, intended to remove damaged subcellular substrates and maintain mitochondrial homeostasis. Autophagy serves a protective or adaptive function in the pathogenesis of disease, including metabolic or mitochondrial dysfunction, protein aggregation, inflammation, and oxidative stress. Consistently, it plays an essential role in maintaining the metabolic homeostasis of lung tissues in chronic respiratory diseases. Therefore, regulation of autophagy under oxidative stress is critical to cell homeostasis and survival. Recent studies have also shown that autophagy profoundly affects inflammation and the immune system, impacting pathogen clearance, cytokine regulation, and antigen presentation. Hence, autophagy plays a key role in human diseases associated with pro-oxidative or pro-inflammatory states. Oxidative stress is a complex phenomenon involved in the physiology and pathophysiology of many lung diseases, and a number of studies have shown that the interaction between ROS and autophagy is closely associated with the development of many lung diseases, including COPD. In this review, we presented an overview of the interplay between autophagy and oxidative stress and focused on the regulation of autophagy under oxidative stress in patients with COPD. Oxidative stress due to CS and environmental pollution plays an essential role in lung inflammation by upregulating redox-sensitive transcription factors, the induction of autophagy as well as unfolded protein response. Interestingly, ROS-induced autophagy can be both a cytoprotective mechanism alleviating oxidative stress and a destructive process. The regulation of autophagy under oxidative stress plays an essential and complex role in the pathogenesis of COPD, but the signaling pathways involved and their molecular effects remain to be defined. Studies have shown that oxidizing agents, hypoxia, and proinflammatory drugs that cause lung damage can activate autophagy, but there have been few studies on lung cells or human lung diseases to date. Appropriate modulation of autophagy is crucial for the development of new treatment strategies for COPD involving oxidative stress. Future studies might include drug screening for molecules that inhibit or induce autophagy, as well as developing autophagy inhibitors or activators and antioxidants e. The paper was conceptualized, reviewed and edited by QZ and RZ, and XZ collected the literature and prepared the original draft. All the authors have read and agreed to publish the manuscript. This study was supported by the grants from Key Laboratory of Intelligent Computing in Medical Image, Northeastern University, Ministry of Education No. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Aggarwal S. Differential regulation of autophagy and mitophagy in pulmonary diseases. Lung Cell. PubMed Abstract CrossRef Full Text Google Scholar. Ahmad T. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: Implications for chronic obstructive pulmonary disease. FASEB J. Al Ghouleh I. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic. TLR4 deficiency promotes autophagy during cigarette smoke-induced pulmonary emphysema. Araya J. PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy 15 3 , — Bae S. Sestrins activate Nrf2 by promoting pdependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. Bellot G. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Brest P. Autophagy and crohn's disease: At the crossroads of infection, inflammation, immunity, and cancer. Burman C. Regulation of autophagy by phosphatidylinositol 3-phosphate. FEBS Lett. Button R. Accumulation of autophagosomes confers cytotoxicity. Cao W. An overview of autophagy: Mechanism, regulation and research progress. Cancer 3 , — Chan E. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. Chang Y. Cell 20 7 , — Chen S. Emerging roles of sestrins in neurodegenerative diseases: Counteracting oxidative stress and beyond. Chen Y. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. Cell Sci. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. Chen Z. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One 3 10 , e Autophagy protein microtubule-associated protein 1 light chain-3B LC3B activates extrinsic apoptosis during cigarette smoke-induced emphysema. Autophagy is essential for ultrafine particle-induced inflammation and mucus hyperproduction in airway epithelium. Autophagy 12 2 , — Church D. Free-radical chemistry of cigarette smoke and its toxicological implications. Health Perspect. Clark S. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. Corona Velazquez A. So many roads: The multifaceted regulation of autophagy induction. Dagda R. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. Dan Dunn J. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. de Cabo R. The search for antiaging interventions: From elixirs to fasting regimens. Cell 7 , — De Duve C. Tissue fractionation studies. Intracellular distribution patterns of enzymes in rat-liver tissue. Deter R. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. Cell Biol. Dodson M. KEAP1—NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Drost E. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 60 4 , — Egan D. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7 6 , — El-Khamisy S. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy Nature , — Eskelinen E. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Acta 4 , — Eun S. PEX5 regulates autophagy via the mTORC1-TFEB axis during starvation. Feng H. Vitro Cell. Rosiglitazone ameliorated airway inflammation induced by cigarette smoke via inhibiting the M1 macrophage polarization by activating PPARγ and RXRα. Filomeni G. Oxidative stress and autophagy: The clash between damage and metabolic needs. Fogel A. Role of membrane association and Atgdependent phosphorylation in beclinmediated autophagy. Fujii S. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology 1 5 , — Galati S. Autophagy: A player in response to oxidative stress and DNA damage. Gegg M. Ghisalberti C. Soft TCPTP agonism-novel target to rescue airway epithelial integrity by exogenous spermidine. Ghosh A. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging Albany NY 8 10 , — Gottlieb R. Mitochondrial turnover in the heart. Acta 7 , — Han D. Sodium tanshinone IIA sulfonate protects ARPE cells against oxidative stress by inhibiting autophagy and apoptosis. Hanada T. The AtgAtg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. Harding T. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. Harper J. Building and decoding ubiquitin chains for mitophagy. Harris H. Control of autophagy as a therapy for neurodegenerative disease. Hasnat M. Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: A novel mechanism in triptolide-induced hepatotoxicity. Regulation mechanisms and signaling pathways of autophagy. The Beclin 1 interactome. Hoffmann R. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Holguin F. Oxidative stress in airway diseases. Hosokawa N. Nutrient-dependent mTORC1 association with the ULK1-AtgFIP complex required for autophagy. Hou H. Elastase induces lung epithelial cell autophagy through placental growth factor: A new insight of emphysema pathogenesis. Autophagy 10 9 , — Hou Y. Sestrin2 protects dopaminergic cells against rotenone toxicity through AMPK-dependent autophagy activation. Hurley J. Mechanisms of autophagy initiation. Hwang J. Cigarette smoke-induced autophagy is regulated by SIRT1-PARPdependent mechanism: Implication in pathogenesis of COPD. Ichimura Y. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Cell 51 5 , — Itakura E. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Cell 19 12 , — Ito S. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11 3 , — Jain A. Jiang Y. Mitochondrial alterations during oxidative stress in chronic obstructive pulmonary disease. Jung C. mTOR regulation of autophagy. Kageyama S. Kang S. Autophagy-related ATG 11, ATG9 and the phosphatidylinositol 3-kinase control ATG2-mediated formation of autophagosomes in Arabidopsis. Plant Cell Rep. Kaspar J. Nrf2:INrf2 Keap1 signaling in oxidative stress. Kelekar A. Kiffin R. Oxidative stress and autophagy. Redox Signal. Kim J. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Kirkham P. Oxidative stress in COPD. Chest 1 , — Klionsky D. Autophagy: From phenomenology to molecular understanding in less than a decade. Knobloch J. Resveratrol impairs the release of steroid-resistant inflammatory cytokines from human airway smooth muscle cells in chronic obstructive pulmonary disease. Kobayashi A. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Komatsu M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Kubli D. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. Lee J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Levine B. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Cell 6 4 , — Imperfect interface of Beclin1 coiled-coil domain regulates homodimer and heterodimer formation with Atg14L and UVRAG. Liang C. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Liu Y. Autosis and autophagic cell death: The dark side of autophagy. Loffler A. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 7 7 , — Cigarette smoke promotes COPD by activating platelet-activating factor receptor and inducing neutrophil autophagic death in mice. Oncotarget 8 43 , — Mack H. AMPK-dependent phosphorylation of ULK1 regulates ATG9 localization. Autophagy 8 8 , — Maiuri M. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 8 10 , — Martens S. Molecular mechanisms of selective autophagy. Mazure N. Hypoxia-induced autophagy: Cell death or cell survival? Mercado N. Accelerated ageing of the lung in COPD: New concepts. Li L-H, Peng W-N, Deng Y, Li J-J, Tian X-R. Moon J-H, Jeong J-K, Hong J-M, Seol J-W, Park S-Y. Inhibition of autophagy by captopril attenuates prion peptide-mediated neuronal apoptosis via AMPK activation. Mol Neurobiol. Cai Z, Yan L-J, Li K, Quazi SH, Zhao B. Weng M-H, Chen S-Y, Li Z-Y, Yen G-C. Free Radic Biol Med. Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, et al. Histol Histopathol. Ludtmann MHR, Abramov AY. Jankovic J, Tan EK. J Neurol Neurosurg Psychiatry Res. Inamdar NN, Arulmozhi DK, Tandon A, Bodhankar SL. Curr Neuropharmacol. Dagda RK, Zhu J, Chu CT. Lu J, Wu M, Yue Z. Autophagy: biology and diseases. Butler D, Bahr BA. Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Chatta GS, Price TH, Stratton JR, Dale DC. Aging and marrow neutrophil reserves. J Am Geriatr Soc. Zhuang X-X, Wang S-F, Tan Y, Song J-X, Zhu Z, Wang Z-Y, et al. Cell Death Dis. Ning B, Zhang Q, Wang N, Deng M, Fang Y. Neurochem Res. Oh SE, Mouradian MM. Regulation of signal transduction by DJ Ebert AD, Beres AJ, Barber AE, Svendsen CN. Exp Neurol. Wang X-W, Yuan L-J, Yang Y, Zhang M, Chen W-F. Am J Physiol Endocrinol Metab. Zhu J, Gao W, Shan X, Wang C, Wang H, Shao Z, et al. Brain Res. Zhang L, Zhang L, Li L, Hölscher C. Mao K, Chen J, Yu H, Li H, Ren Y, Wu X, et al. Aging Cell. Lin C-H, Wei P-C, Chen C-M, Huang Y-T, Lin J-L, Lo Y-S, et al. Guo Y-L, Duan W-J, Lu D-H, Ma X-H, Li X-X, Li Z, et al. He X-H, Lin F, Qin Z-H. Current understanding on the pathogenesis of polyglutamine diseases. Neurosci Bull. Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RAV, et al. Sci Transl Med. Cortes CJ, La Spada AR. Drug Discov Today. An T, Shi P, Duan W, Zhang S, Yuan P, Li Z, et al. Oxidative stress and autophagic alteration in brainstem of SOD1-G93A mouse model of ALS. Deng Z, Sheehan P, Chen S, Yue Z. Mol Neurodegener. Ma L, Herren AW, Espinal G, Randol J, McLaughlin B, Martinez-Cerdeño V, et al. Acta Neuropathol Commun. Wardman JH, Henriksen EE, Marthaler AG, Nielsen JE, Nielsen TT. Enhancement of autophagy and solubilization of ataxin-2 alleviate apoptosis in spinocerebellar ataxia type 2 patient cells. Morani F, Doccini S, Sirica R, Paterno M, Pezzini F, Ricca I, et al. Functional transcriptome analysis in ARSACS KO cell model reveals a role of sacsin in autophagy. Sci Rep. Ye B, Wang Q, Hu H, Shen Y, Fan C, Chen P, et al. Restoring autophagic flux attenuates cochlear spiral ganglion neuron degeneration by promoting TFEB nuclear translocation via inhibiting MTOR. Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. Yamada D, Kawabe K, Tosa I, Tsukamoto S, Nakazato R, Kou M, et al. Inhibition of the glutamine transporter SNAT1 confers neuroprotection in mice by modulating the mTOR-autophagy system. Commun Biol. Mackenzie B, Schäfer MKH, Erickson JD, Hediger MA, Weihe E, Varoqui H. Functional properties and cellular distribution of the system A glutamine transporter SNAT1 support specialized roles in central neurons. J Biol Chem. Hägglund MGA, Sreedharan S, Nilsson VCO, Shaik JHA, Almkvist IM, Bäcklin S, et al. Identification of SLC38A7 SNAT7 protein as a glutamine transporter expressed in neurons. Bagchi S, Baomar HA, Al-Walai S, Al-Sadi S, Fredriksson R. Histological analysis of SLC38A6 SNAT6 expression in mouse brain shows selective expression in excitatory neurons with high expression in the synapses. PLoS ONE. Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, et al. Tsc1 hamartin confers neuroprotection against ischemia by inducing autophagy. Nat Med. Liu Y, Xue X, Zhang H, Che X, Luo J, Wang P, et al. Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Aredia F, Scovassi AI. A new function for miRNAs as regulators of autophagy. Future Med Chem. Wang Y, Meng C, Zhang J, Wu J, Zhao J. Int Immunopharmacol. Fang C, Gu L, Smerin D, Mao S, Xiong X. The interrelation between reactive oxygen species and autophagy in neurological disorders. Liu S, Su Y, Sun B, Hao R, Pan S, Gao X, et al. Su SH, Wang YQ, Wu YF, Wang DP, Lin Q, Hai J. Behav Brain Res. Su SH, Wu YF, Lin Q, Yu F, Hai J. Cannabinoid receptor agonist WIN55,—2 and fatty acid amide hydrolase inhibitor URB suppress chronic cerebral hypoperfusion-induced neuronal apoptosis by inhibiting c-Jun N-terminal kinase signaling. Su SH, Wu YF, Lin Q, Hai J. Cannabinoid receptor agonist WIN55,—2 and fatty acid amide hydrolase inhibitor URB ameliorate neuroinflammatory responses in chronic cerebral hypoperfusion model by blocking NF-κB pathways. Naunyn Schmiedebergs Arch Pharmacol. Wang D, Lin Q, Su S, Liu K, Wu Y, Hai J. URB improves cognitive impairment induced by chronic cerebral hypoperfusion by inhibiting mTOR-dependent autophagy. Zhang D, Zhu D, Wang F, Zhu J-C, Zhai X, Yuan Y, et al. Therapeutic effect of regulating autophagy in spinal cord injury: a network meta-analysis of direct and indirect comparisons. Zhu Y, Tang Q, Wang G, Han R. Tanshinone IIA protects hippocampal neuronal cells from reactive oxygen species through changes in autophagy and activation of phosphatidylinositol 3-kinase, protein kinas B, and mechanistic target of rapamycin pathways. Curr Neurovasc Res. Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: a comprehensive review. Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Žugich J. Key role of T cell defects in age-related vulnerability to West Nile virusT cell defects and age-related vulnerability to WNV. J Exp Med. Khandia R, Dadar M, Munjal A, Dhama K, Karthik K, Tiwari R, et al. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: current knowledge and prospects for disease prevention, novel drug design, and therapy. Krishnamurthy S, Konstantinou EK, Young LH, Gold DA, Saeij JPJ. The human immune response to Toxoplasma: autophagy versus cell death. PLoS Pathog. Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Jiang G-M, Tan Y, Wang H, Peng L, Chen H-T, Meng X-J, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. Sun C-Y, Zhang Q-Y, Zheng G-J, Feng B. Autophagy and its potent modulators from phytochemicals in cancer treatment. Cancer Chemother Pharmacol. Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Role of autophagy in osteosarcoma. J Bone Oncol. Wang P, Zhao ZQ, Guo SB, Yang TY, Chang ZQ, Li DH, et al. Roles of microRNA in suppressing proliferation and promoting sensitivity of osteosarcoma cells via metadherin-mediated autophagy. Orthop Surg. Jamali Z, Taheri-Anganeh M, Shabaninejad Z, Keshavarzi A, Taghizadeh H, Razavi ZS, et al. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. Zhu Z, Zhang P, Li N, Kiang KMY, Cheng SY, Wong VKW, et al. Lovastatin enhances cytotoxicity of temozolomide via impairing autophagic flux in glioblastoma cells. Biomed Res Int. Mora R, Régnier-Vigouroux A. Shen S, Zhang Y, Wang Z, Zhang R, Gong X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int J Biol Sci. Nazim UM, Jeong J-K, Seol J-W, Hur J, Eo S-K, Lee J-H, et al. Inhibition of the autophagy flux by gingerol enhances TRAIL-induced tumor cell death. Oncol Rep. Rasheduzzaman M, Park S-Y. Antihypertensive drug-candesartan attenuates TRAIL resistance in human lung cancer via AMPK-mediated inhibition of autophagy flux. Exp Cell Res. Hou HH, Pan HJ, Liao WY, Lee CH, Yu CJ. Autophagy in fibroblasts induced by cigarette smoke extract promotes invasion in lung cancer cells. Int J Cancer. Zhang L, Zhang J, Chen L, Wang J. Autophagy in human skin squamous cell carcinoma: Inhibition by 3-MA enhances the effect of 5-FU-induced chemotherapy sensitivity. Zhang L, Ji Z, Zhang J, Yang S. Photodynamic therapy enhances skin cancer chemotherapy effects through autophagy regulation. Photodiagnosis Photodyn Ther. Zhang Y, Du Y, Le W, Wang K, Kieffer N, Zhang J. Redox control of the survival of healthy and diseased cells. Mancias JD, Kimmelman AC. Mechanisms of selective autophagy in normal physiology and cancer. J Mol Biol. Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. Yun CW, Lee SH. The roles of autophagy in cancer. Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. Hazeldine J, Lord JM, Hampson P. Immunesenescence and inflammaging: a contributory factor in the poor outcome of the geriatric trauma patient. Solana R, Pawelec G, Tarazona R. Aging and Innate Immunity. Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, et al. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol. Lin S-C, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Pourbagher-Shahri AM, Farkhondeh T, Talebi M, Kopustinskiene DM, Samarghandian S, Bernatoniene J. An overview of NO signaling pathways in aging. Lin X-T, Zheng X-B, Fan D-J, Yao Q-Q, Hu J-C, Lian L, et al. Inflamm Bowel Dis. Kato H, Perl A. Blockade of treg cell differentiation and function by the interleukin—mechanistic target of rapamycin axis via suppression of autophagy in patients with systemic lupus erythematosus. Arthritis Rheumatol. Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Xie W, Zhou J. Aberrant regulation of autophagy in mammalian diseases. Biol Lett. Meng Y, Pan M, Zheng B, Chen Y, Li W, Yang Q, et al. Autophagy attenuates angiotensin II-induced pulmonary fibrosis by inhibiting redox imbalance-mediated NOD-like receptor family pyrin domain containing 3 inflammasome activation. Yin Y, Zong R, Bao X, Zheng X, Cui H, Liu Z, et al. Oxidative stress suppresses cellular autophagy in corneal epithelium. Invest Ophthalmol Vis Sci. Farkhondeh T, Samarghandian S, Azimi-Nezhad M. The role of arsenic in obesity and diabetes. Qian Q, Zhang Z, Orwig A, Chen S, Ding W-X, Xu Y, et al. S-Nitrosoglutathione Reductase Dysfunction Contributes to Obesity-Associated Hepatic Insulin Resistance via Regulating Autophagy. Zheng R-H, Zhang W-W, Ji Y-N, Bai X-J, Yan C-P, Wang J, et al. Lenoir O, Tharaux P-L, Huber TB. Autophagy in kidney disease and aging: lessons from rodent models. Kidney Int. Allaire M, Rautou P-E, Codogno P, Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol. Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. Yazdani E, Talebi M, Zarshenas M, Moein M. Evaluation of possible antioxidant activities of barberry solid formulation, a selected formulation from Traditional Persian Medicine TPM via various procedures. Ke P-Y. Diverse functions of autophagy in liver physiology and liver diseases. Int J Mol Med Sci. Yin X, Zhou C, Li J, Liu R, Shi B, Yuan Q, et al. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. Yang Y, Klionsky DJ. Autophagy and disease: unanswered questions. Cell Death Differ. Ebrahimi-Fakhari D, Saffari A, Wahlster L, Lu J, Byrne S, Hoffmann GF, et al. Congenital disorders of autophagy: an emerging novel class of inborn errors of neuro-metabolism. Ebrahimi-Fakhari D. Congenital disorders of autophagy: what a pediatric neurologist should know. Forlenza OV, De-Paula VDJR, Diniz BSO. ACS Chem Neurosci. Brose RD, Lehrmann E, Zhang Y, Reeves RH, Smith KD, Mattson MP. Neurobiol Aging. Çelik H, Karahan H, Kelicen-Uğur P. Effect of atorvastatin on Aβ1—induced alteration of SESN2, SIRT1, LC3II and TPP1 protein expressions in neuronal cell cultures. Phiwchai I, Chariyarangsitham W, Phatruengdet T, Pilapong C. Ferric-tannic nanoparticles increase neuronal cellular clearance. Wang C, Zhang X, Teng Z, Zhang T, Li Y. Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y. Exp Gerontol. Wang H, Jiang T, Li W, Gao N, Zhang T. Toxicol Lett. Zhang Z, Wang X, Di Zhang YL, Li L. Aging Albany NY. Wang X, Shan P, Liu A, Ma L, Lu M, Jiang W, et al. Polydatin prevents A beta-induced neuron cytotoxicity via enhancing autophagy and decreasing oxidative stress. Int J Clin Exp Med. Luengo E, Buendia I, Fernández-Mendívil C, Trigo-Alonso P, Negredo P, Michalska P, et al. Pharmacological doses of melatonin impede cognitive decline in tau-related Alzheimer models, once tauopathy is initiated, by restoring the autophagic flux. J Pineal Res. Gugliandolo A, Chiricosta L, Silvestro S, Bramanti P, Mazzon E. Brain Sci. Rigacci S, Miceli C, Nediani C, Berti A, Cascella R, Pantano D, et al. Cordero JG, García-Escudero R, Avila J, Gargini R, García-Escudero V. Sharma C, Kang SC. Garcinol pacifies acrylamide induced cognitive impairments, neuroinflammation and neuronal apoptosis by modulating GSK signaling and activation of pCREB by regulating cathepsin B in the brain of zebrafish larvae. Food Chem Toxicol. Liu J, Su H, Qu Q-M. Carnosic acid prevents beta-amyloid-induced injury in human neuroblastoma sh-sy5y cells via the induction of autophagy. Xue Z, Guo Y, Zhang S, Huang L, He Y, Fang R, et al. Yuan H, Jiang C, Zhao J, Zhao Y, Zhang Y, Xu Y, et al. Euxanthone Attenuates Aβ 1—Induced Oxidative Stress and Apoptosis by Triggering Autophagy. J Mol Neurosci. Al Rihani SB, Darakjian LI, Kaddoumi A. Oleocanthal-rich extra-virgin olive oil restores the blood—brain barrier function through NLRP3 inflammasome inhibition simultaneously with autophagy induction in TgSwDI mice. Ren Z-L, Wang C-D, Wang T, Ding H, Zhou M, Yang N, et al. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Sportelli C, Urso D, Jenner P, Chaudhuri K. Front Neurol. Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2 and autophagy pathways. Wang B, Su C-J, Liu T-T, Zhou Y, Feng Y, Huang Y, et al. Front Mol Neurosci. Ngwa HA, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol. Ding Y, Kong D, Zhou T, Xin C, Xu J, Wang Q, et al. Neuromol Med. Bai H, Ding Y, Li X, Kong D, Xin C, Yang X, et al. Neurochem Int. Yang G, Li J, Cai Y, Yang Z, Li R, Fu W. Glycyrrhizic acid alleviates 6-hydroxydopamine and corticosterone-induced neurotoxicity in SH-SY5Y cells through modulating autophagy. Zhou L, Cheng Y. Zeng R, Zhou Q, Zhang W, Fu X, Wu Q, Lu Y, et al. Icariin-mediated activation of autophagy confers protective effect on rotenone induced neurotoxicity in vivo and in vitro. Toxicol Rep. Wei C-C, Chang C-H, Liao VHC. Anti-Parkinsonian effects of β-amyrin are regulated via LGG-1 involved autophagy pathway in Caenorhabditis elegans. Filomeni G, Graziani I, De Zio D, Dini L, Centonze D, Rotilio G, et al. Liu P, Li Y, Yang W, Liu D, Ji X, Chi T, et al. Chang C-C, Lin T-C, Ho H-L, Kuo C-Y, Li H-H, Korolenko TA, et al. GLP-1 analogue liraglutide attenuates mutant huntingtin-induced neurotoxicity by restoration of neuronal insulin signaling. Fernandez-Estevez MA, Casarejos MJ, López Sendon J, Garcia Caldentey J, Ruiz C, Gomez A, et al. Vidoni C, Secomandi E, Castiglioni A, Melone MAB, Isidoro C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Cordeiro LM, Machado ML, da Silva AF, Baptista FBO, da Silveira TL, Soares FAA, et al. Yang J, Bridges K, Chen KY, Liu AYC. Riluzole increases the amount of latent HSF1 for an amplified heat shock response and cytoprotection. Ueda T, Ito T, Kurita H, Inden M, Hozumi I. p-Coumaric acid has protective effects against mutant copper—zinc superoxide dismutase 1 via the activation of autophagy in N2a cells. Lv B, Jiang X-M, Wang D-W, Chen J, Han D-F, Liu X-L. Protective effects and mechanisms of action of ulinastatin against cerebral ischemia-reperfusion injury. Wang R, Liu Y-Y, Liu X-Y, Jia S-W, Zhao J, Cui D, et al. Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell Physiol Biochem. Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D, et al. J Neuropathol Exp Neurol. Yang B, Zang L-E, Cui J-W, Zhang M-Y, Ma X, Wei L-L. Melatonin plays a protective role by regulating miRa-5p-NRSF and JAK2-STAT3 pathway to improve autophagy, inflammation and oxidative stress of cerebral ischemia-reperfusion injury. Drug Des Devel Ther. Yu Y, Wu X, Pu J, Luo P, Ma W, Wang J, et al. Biochem Biophys Res Commun. Yu F, Xue W, Dong L, Hu X, Huang D, Wang K. Evid Based Complement Alternat Med. Yang H, Li L, Zhou K, Wang Y, Guan T, Chai C, et al. Pharm Biol. Xu B, Zhu L, Chu J, Ma Z, Fu Q, Wei W, et al. Esculetin improves cognitive impairments induced by transient cerebral ischaemia and reperfusion in mice via regulation of mitochondrial fragmentation and mitophagy. Wang N, He J, Pan C, Wang J, Ma M, Shi X, et al. Front Neurosci. Wojdasiewicz P, Poniatowski ŁA, Turczyn P, Frasuńska J, Paradowska-Gorycka A, Tarnacka B. Significance of omega-3 fatty acids in the prophylaxis and treatment after spinal cord injury in rodent models. Mediators Inflamm. Zhou KL, Chen DH, Jin HM, Wu K, Wang XY, Xu HZ, et al. Effects of calcitriol on experimental spinal cord injury in rats. Spinal Cord. Wu Y-L, Chang J-C, Lin W-Y, Li C-C, Hsieh M, Chen H-W, et al. Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Lin C-H, Wu Y-R, Yang J-M, Chen W-L, Chao C-Y, Chen I, et al. Novel lactulose and melibiose targeting autophagy to reduce polyQ aggregation in cell models of spinocerebellar ataxia 3. CNS Neurol Disord Drug Targets. Download references. Department of Pharmacognosy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Department of Pharmaceutical Biotechnology, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Department of Chemistry and Biochemistry, University of Texas at Arlington, Arlington, TX, , USA. Viatris Pharmaceuticals Inc, Research Plaza, San Antonio, TX, , USA. Medical Toxicology and Drug Abuse Research Center MTDRC , Birjand University of Medical Sciences, Birjand, Iran. Faculty of Pharmacy, Birjand University of Medical Sciences, Birjand, Iran. Noncommunicable Diseases Research Center, Neyshabur University of Medical Sciences, Neyshabur, Iran. You can also search for this author in PubMed Google Scholar. All authors contributed to the manuscript. Conceptualization, MT and SS; validation investigation, resources, data extraction, and writing, all authors; review and editing, MT and SS, All authors read and approved the final manuscript. Correspondence to Saeed Samarghandian. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Talebi, M. et al. The interplay between oxidative stress and autophagy: focus on the development of neurological diseases. Behav Brain Funct 18 , 3 Download citation. Received : 11 May Accepted : 17 January Published : 29 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract Regarding the epidemiological studies, neurological dysfunctions caused by cerebral ischemia or neurodegenerative diseases NDDs have been considered a pointed matter. Molecular regulation of autophagy The molecular mechanisms attributed to the occurrence of autophagy are widely researched. The relationship between reactive oxygen species and autophagy ROS are a large group of highly reactive-oxygen-containing species that comprise free radicals, oxygen anions, and hydrogen peroxide and are known for their short lives and high reaction desire [ 26 , 27 ]. Full size image. Autophagy and ROS in neurological diseases There are many relations observed between neurodegenerative disorders and autophagy. Table 1 Summary of compounds involved in regulation activation or inactivation of autophagy process of neurological diseases Full size table. Therapeutic implications of autophagy dysregulation The induction of therapeutic autophagy acts as a survival mechanism. Conclusion In summary, regarding the emerging emphasis on cellular autophagy, a comprehensive literature overview was conducted to discuss the pharmacological aspects of autophagy, focusing on its interplay with oxidative stress in neurological disorders. References Wang M-M, Feng Y-S, Yang S-D, Xing Y, Zhang J, Dong F, et al. CAS PubMed PubMed Central Google Scholar Farkhondeh T, Samarghandian S, Pourbagher-Shahri AM, Sedaghat M. CAS PubMed Google Scholar Klionsky DJ. CAS PubMed Google Scholar Wang S-Y, Yu Q-J, Zhang R-D, Liu B. CAS PubMed Google Scholar Zhang J. CAS PubMed PubMed Central Google Scholar Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, et al. CAS Google Scholar Rahman MA, Rahman MR, Zaman T, Uddin MS, Islam R, Abdel-Daim MM, et al. CAS PubMed Google Scholar Kesidou E, Lagoudaki R, Touloumi O, Poulatsidou K-N, Simeonidou C. CAS PubMed PubMed Central Google Scholar Batatinha HAP, Diniz TA, de Souza Teixeira AA, Krüger K, Rosa-Neto JC. CAS Google Scholar Mizushima N, Levine B, Cuervo AM, Klionsky DJ. CAS PubMed PubMed Central Google Scholar Tang C, Livingston MJ, Liu Z, Dong Z. CAS PubMed PubMed Central Google Scholar Handy DE, Loscalzo J. CAS PubMed PubMed Central Google Scholar Yun HR, Jo YH, Kim J, Shin Y, Kim SS, Choi TG. CAS PubMed Central Google Scholar Hajzadeh MA, Rajaei Z, Shafiee S, Alavinejhad A, Samarghandian S, Ahmadi M. Google Scholar Samarghandian S, Samini F, Azimi-Nezhad M, Farkhondeh T. Google Scholar Singh SS, Vats S, Chia AY-Q, Tan TZ, Deng S, Ong MS, et al. CAS PubMed Google Scholar Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. CAS PubMed PubMed Central Google Scholar Grumati P, Bonaldo P. PubMed PubMed Central Google Scholar Desideri E, Vegliante R, Cardaci S, Nepravishta R, Paci M, Ciriolo MR. CAS PubMed PubMed Central Google Scholar Du J, Liang Y, Xu F, Sun B, Wang Z. CAS PubMed Google Scholar Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian S. CAS PubMed Google Scholar Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian S. CAS PubMed Google Scholar Ashrafizadeh M, Zarrabi A, Orouei S, Hushmandi K, Hakimi A, Zabolian A, et al. Article PubMed Google Scholar Desai S, Juncker M, Kim C. CAS Google Scholar Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. CAS PubMed PubMed Central Google Scholar Talebi M, Mojab F. Article Google Scholar Talebi M, İlgün S, Ebrahimi V, Talebi M, Farkhondeh T, Ebrahimi H, et al. Article PubMed Google Scholar Talebi M, Kakouri E, Talebi M, Tarantilis PA, Farkhondeh T, İlgün S, et al. CAS PubMed Google Scholar Bonomini F, Rodella LF, Rezzani R. PubMed PubMed Central Google Scholar Dewaele M, Maes H, Agostinis P. CAS PubMed Google Scholar Fransen M, Lismont C. CAS PubMed Google Scholar Talebi M, Talebi M, Kakouri E, Farkhondeh T, Pourbagher-Shahri AM, Tarantilis PA, et al. CAS PubMed Google Scholar Lan A-P, Chen J, Zhao Y, Chai Z, Hu Y. PubMed Google Scholar Lipton JO, Sahin M. CAS PubMed PubMed Central Google Scholar Alexander A, Cai S-L, Kim J, Nanez A, Sahin M, MacLean KH, et al. CAS PubMed PubMed Central Google Scholar Kongara S, Karantza V. PubMed PubMed Central Google Scholar Azad MB, Chen Y, Gibson SB. CAS PubMed Google Scholar Samarghandian S, Azimi-Nezhad M, Farkhondeh T. CAS PubMed Google Scholar Talebi M, Talebi M, Farkhondeh T, Mishra G, İlgün S, Samarghandian S. CAS PubMed Google Scholar Pajares M, Jiménez-Moreno N, García-Yagüe ÁJ, Escoll M, de Ceballos ML, Van Leuven F, et al. CAS PubMed PubMed Central Google Scholar Zhao RZ, Jiang S, Zhang L, Yu ZB. CAS PubMed PubMed Central Google Scholar Dong X-X, Wang Y, Qin Z-H. CAS PubMed PubMed Central Google Scholar Funderburk SF, Marcellino BK, Yue Z. PubMed PubMed Central Google Scholar Essick EE, Sam F. Google Scholar Cheng W-T, Guo Z-X, Lin C-A, Lin M-Y, Tung L-C, Fang K. Article PubMed PubMed Central Google Scholar Choi KC, Kim SH, Ha JY, Kim ST, Son JH. CAS PubMed Google Scholar Talebi M, Talebi M, Samarghandian S. CAS Google Scholar Uddin MS, Kabir MT, Tewari D, Mamun AA, Mathew B, Aleya L, et al. CAS PubMed Google Scholar Talebi M, Esmaeeli H, Talebi M, Farkhondeh T, Samarghandian S. Article Google Scholar Duncan RS, Song B, Koulen P. PubMed Central Google Scholar Uddin M, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, et al. Google Scholar Gorantla NV, Chinnathambi S. Google Scholar Di Domenico F, Barone E, Perluigi M, Butterfield DA. PubMed Google Scholar Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. CAS PubMed PubMed Central Google Scholar Uddin MS, Rahman MA, Kabir MT, Behl T, Mathew B, Perveen A, et al. CAS PubMed Google Scholar Huang J-L, Su M, Wu D-P. CAS PubMed Google Scholar Kim J, Yoon H, Kim J. CAS Google Scholar Du F, Yu Q, Yan S, Hu G, Lue L-F, Walker DG, et al. PubMed PubMed Central Google Scholar Li L. CAS PubMed Google Scholar Zheng X, Wang W, Liu R, Huang H, Zhang R, Sun L. CAS PubMed PubMed Central Google Scholar Agrawal I, Jha S. CAS PubMed PubMed Central Google Scholar Zhang Y-d, Zhao J-j. CAS PubMed Google Scholar Omata Y, Lim Y-M, Akao Y, Tsuda L. PubMed PubMed Central Google Scholar Li L-H, Peng W-N, Deng Y, Li J-J, Tian X-R. PubMed Google Scholar Moon J-H, Jeong J-K, Hong J-M, Seol J-W, Park S-Y. CAS PubMed Google Scholar Cai Z, Yan L-J, Li K, Quazi SH, Zhao B. CAS PubMed Google Scholar Weng M-H, Chen S-Y, Li Z-Y, Yen G-C. CAS PubMed Google Scholar Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, et al. CAS PubMed Google Scholar Ludtmann MHR, Abramov AY. CAS PubMed Google Scholar Jankovic J, Tan EK. Google Scholar Inamdar NN, Arulmozhi DK, Tandon A, Bodhankar SL. CAS PubMed PubMed Central Google Scholar Dagda RK, Zhu J, Chu CT. CAS PubMed PubMed Central Google Scholar Lu J, Wu M, Yue Z. Google Scholar Butler D, Bahr BA. CAS PubMed Google Scholar Chatta GS, Price TH, Stratton JR, Dale DC. CAS PubMed Google Scholar Zhuang X-X, Wang S-F, Tan Y, Song J-X, Zhu Z, Wang Z-Y, et al. Google Scholar Ning B, Zhang Q, Wang N, Deng M, Fang Y. CAS PubMed Google Scholar Oh SE, Mouradian MM. Google Scholar Ebert AD, Beres AJ, Barber AE, Svendsen CN. CAS PubMed Google Scholar Wang X-W, Yuan L-J, Yang Y, Zhang M, Chen W-F. CAS PubMed Google Scholar Zhu J, Gao W, Shan X, Wang C, Wang H, Shao Z, et al. CAS PubMed Google Scholar Zhang L, Zhang L, Li L, Hölscher C. CAS PubMed Google Scholar Mao K, Chen J, Yu H, Li H, Ren Y, Wu X, et al. CAS PubMed PubMed Central Google Scholar Lin C-H, Wei P-C, Chen C-M, Huang Y-T, Lin J-L, Lo Y-S, et al. Article PubMed PubMed Central Google Scholar Guo Y-L, Duan W-J, Lu D-H, Ma X-H, Li X-X, Li Z, et al. CAS PubMed Google Scholar He X-H, Lin F, Qin Z-H. CAS PubMed PubMed Central Google Scholar Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RAV, et al. PubMed PubMed Central Google Scholar Cortes CJ, La Spada AR. CAS PubMed PubMed Central Google Scholar An T, Shi P, Duan W, Zhang S, Yuan P, Li Z, et al. CAS PubMed Google Scholar Deng Z, Sheehan P, Chen S, Yue Z. Google Scholar Ma L, Herren AW, Espinal G, Randol J, McLaughlin B, Martinez-Cerdeño V, et al. Google Scholar Wardman JH, Henriksen EE, Marthaler AG, Nielsen JE, Nielsen TT. CAS PubMed Google Scholar Morani F, Doccini S, Sirica R, Paterno M, Pezzini F, Ricca I, et al. CAS Google Scholar Ye B, Wang Q, Hu H, Shen Y, Fan C, Chen P, et al. CAS PubMed PubMed Central Google Scholar Lin L, Yee SW, Kim RB, Giacomini KM. CAS PubMed PubMed Central Google Scholar Yamada D, Kawabe K, Tosa I, Tsukamoto S, Nakazato R, Kou M, et al. CAS Google Scholar Mackenzie B, Schäfer MKH, Erickson JD, Hediger MA, Weihe E, Varoqui H. CAS PubMed Google Scholar Hägglund MGA, Sreedharan S, Nilsson VCO, Shaik JHA, Almkvist IM, Bäcklin S, et al. PubMed PubMed Central Google Scholar Bagchi S, Baomar HA, Al-Walai S, Al-Sadi S, Fredriksson R. PubMed PubMed Central Google Scholar Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, et al. CAS PubMed PubMed Central Google Scholar Liu Y, Xue X, Zhang H, Che X, Luo J, Wang P, et al. PubMed Google Scholar Aredia F, Scovassi AI. CAS PubMed Google Scholar Wang Y, Meng C, Zhang J, Wu J, Zhao J. CAS PubMed Google Scholar Fang C, Gu L, Smerin D, Mao S, Xiong X. Article PubMed PubMed Central Google Scholar Liu S, Su Y, Sun B, Hao R, Pan S, Gao X, et al. CAS PubMed Google Scholar Su SH, Wang YQ, Wu YF, Wang DP, Lin Q, Hai J. CAS PubMed Google Scholar Su SH, Wu YF, Lin Q, Yu F, Hai J. CAS PubMed Google Scholar Su SH, Wu YF, Lin Q, Hai J. CAS PubMed Google Scholar Wang D, Lin Q, Su S, Liu K, Wu Y, Hai J. CAS PubMed Google Scholar Zhang D, Zhu D, Wang F, Zhu J-C, Zhai X, Yuan Y, et al. PubMed Google Scholar Zhu Y, Tang Q, Wang G, Han R. CAS PubMed PubMed Central Google Scholar Saha S, Panigrahi DP, Patil S, Bhutia SK. CAS PubMed Google Scholar Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Žugich J. CAS PubMed PubMed Central Google Scholar Khandia R, Dadar M, Munjal A, Dhama K, Karthik K, Tiwari R, et al. CAS PubMed Central Google Scholar Krishnamurthy S, Konstantinou EK, Young LH, Gold DA, Saeij JPJ. PubMed PubMed Central Google Scholar Rebecca VW, Amaravadi RK. CAS PubMed Google Scholar Jiang G-M, Tan Y, Wang H, Peng L, Chen H-T, Meng X-J, et al. CAS Google Scholar Sun C-Y, Zhang Q-Y, Zheng G-J, Feng B. PubMed Google Scholar Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. PubMed PubMed Central Google Scholar Wang P, Zhao ZQ, Guo SB, Yang TY, Chang ZQ, Li DH, et al. PubMed PubMed Central Google Scholar Jamali Z, Taheri-Anganeh M, Shabaninejad Z, Keshavarzi A, Taghizadeh H, Razavi ZS, et al. CAS PubMed Google Scholar Zhu Z, Zhang P, Li N, Kiang KMY, Cheng SY, Wong VKW, et al. Article PubMed PubMed Central Google Scholar Mora R, Régnier-Vigouroux A. CAS PubMed Google Scholar Shen S, Zhang Y, Wang Z, Zhang R, Gong X. CAS PubMed PubMed Central Google Scholar Nazim UM, Jeong J-K, Seol J-W, Hur J, Eo S-K, Lee J-H, et al. CAS PubMed Google Scholar Rasheduzzaman M, Park S-Y. CAS PubMed Google Scholar Hou HH, Pan HJ, Liao WY, Lee CH, Yu CJ. CAS PubMed Google Scholar Zhang L, Zhang J, Chen L, Wang J. CAS PubMed Google Scholar Zhang L, Ji Z, Zhang J, Yang S. CAS PubMed Google Scholar Zhang Y, Du Y, Le W, Wang K, Kieffer N, Zhang J. CAS PubMed Google Scholar Mancias JD, Kimmelman AC. CAS PubMed PubMed Central Google Scholar Jiang P, Mizushima N. CAS PubMed Google Scholar Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al. CAS PubMed PubMed Central Google Scholar Yun CW, Lee SH. PubMed Central Google Scholar Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. CAS PubMed PubMed Central Google Scholar Hazeldine J, Lord JM, Hampson P. PubMed Google Scholar Solana R, Pawelec G, Tarazona R. CAS PubMed Google Scholar Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, et al. CAS PubMed PubMed Central Google Scholar Lin S-C, Hardie DG. CAS Google Scholar Pourbagher-Shahri AM, Farkhondeh T, Talebi M, Kopustinskiene DM, Samarghandian S, Bernatoniene J. Article PubMed PubMed Central Google Scholar Lin X-T, Zheng X-B, Fan D-J, Yao Q-Q, Hu J-C, Lian L, et al. |
| Oxidative Stress and Autophagy | SpringerLink | Cell Autophagy and oxidative stress 121 Structure of the anx atgatg HORMA heterodimer: an interaction hub within the Oxidarive complex. Autophagy and oxidative stress relays and phosphorylative ans partners abd redox-mediated signaling pathways. Article CAS PubMed PubMed Central Google Scholar Lopez-Rivera E, Jayaraman P, Parikh F, Davies MA, Ekmekcioglu S, Izadmehr S et al. The authors would like to acknowledge the published work that were cited for this review and those that were not cited because of space and our own limitations. |
| Top bar navigation | To determine whether this difference between human transformed cell lines and mouse primary cells was not due to differences between human and mouse cells, the transformed mouse NIH 3T3 fibroblast cell line was treated with H 2 O 2 and 2-ME. The amount of ROS generation, AVO formation and GFP-LC3 vacuoles formation increased and the amount of H 2 O 2 - and 2-ME induced cell death was blocked by 3-MA Supplementary Figure S8. Therefore, unlike in transformed cells, H 2 O 2 and 2-ME preferentially induced apoptotic cell death in nontransformed primary mouse astrocytes. H 2 O 2 and 2-ME fail to induce autophagy in primary mouse astrocytes. Primary mouse astrocytes were treated with H 2 O 2 or 2-ME as indicated. b Autophagy was determined as described above. ii Formation of GFP-LC3 vacuoles dots over a h time course in the absence and presence of NH 4 Cl. iii Beclin-1 expression was determined by western blotting after cells were treated with H 2 O 2 1. d Cell death was determined as previously indicated. Oxidative stress has been shown to induce autophagy under certain conditions such as ischemia and reperfusion. Herein we demonstrated for the first time that using H 2 O 2 and 2-ME to induce oxidative stress caused autophagy-induced cell death in transformed cell line HEK and cancer cell lines U87 and HeLa cells. Blocking autophagosome accumulation through chemical inhibitors or knocking down autophagy genes effectively blocked oxidative stress-induced cell death. Furthermore, blocking ROS generation also effectively blocked autophagy and cell death. In contrast, mouse primary astrocytes following oxidative stress failed to undergo autophagy. This indicates that oxidative stress induces autophagy-mediated cell death in transformed and cancer cells. Autophagic cell death remains controversial since autophagy contributes to cell survival under stress such as starvation. The metabolic toxin arsenic trioxide induced autophagic cell death mediated by upregulation of pro-cell death Bcl-2 family member BNIP3. We have determined that under oxidative stress, autophagy contributes to cell death. Taken together, autophagy could contribute to both cell survival and cell death. Besides autophagy, oxidative stress has been shown to induce apoptotic signaling pathways leading to cell death. This is similar to the effect of arsenic trioxide As 2 O 3 on cell death in human T-lymphocytic leukemia and myelodysplastic syndrome MDS cell lines. This indicates that when H 2 O 2 -or 2-ME-induced apoptotic cell death pathway is blocked, cells preferentially die by the autophagic pathway. Conversely, blockage of autophagosome accumulation failed to significantly alter apoptosis. This suggest that apoptosis occurs independent of autophagy. The amount of oxidative stress-induced cell death was not completely blocked by inhibiting either apoptosis or autophagy. This indicates that there is a third cell death pathway. This third type of cell death could be the necrotic cell death pathway. This form of cell death is passive and causes cellular contents to be released to the extracellular space and often causes inflammation. Reports have shown that when apoptotic or autophagic cell death was blocked, necrotic cell death was observed. Nevertheless, our results indicate that autophagic cell death pathway plays an important role in oxidative stress-induced cell death. Autophagy pathways have been extensively studied. A recent report suggests that ROS could be involved in caspase-independent cell death in macrophage cells. Tiron treatment however failed to completely eliminate oxidative stress-induced ROS generation suggesting that higher levels of ROS or the types of ROS generated might be important in regulating autophagy independent of apoptosis. Indeed, ROS have many effects on cells including DNA damage, mitochondrial dysfunction, activation of signaling pathways and activation of transcription factors leading to upregulation of genes. This agrees with the report by Thorpe et al. The mechanism for ROS-mediated upregulation of beclin-1 and the differences between ROS-induced apoptosis and autophagy will be the focus for further investigation. Cancer cells produce higher levels of ROS than normal cells, and this contributes to cancer progression. Hagen et al. Indeed, overexpression of mitochondrial SOD2 blocks ROS generation and autophagosome accumulation induced by 2-ME. A strategy to sensitize cancer cells to drug-induced apoptosis is to combine an ROS-generating drug with the inhibition of mitochondrial respiration enhancing ROS production and cell death such as combining As 2 O 3 with rotenone an inhibitor of mETC complex I. In addition, autophagy failed to be significantly induced in primary astrocytes under oxidative stress. Thus, targeting ROS generation could selectively induce autophagic cell death in cancer cells. In conclusion, oxidative stress induces autophagy and provides a novel mechanism for oxidative stress-induced cell death that is selective toward transformed and cancer cells. This may lead to new strategies to develop therapeutic drugs that will selectively target cancer cells to undergo autophagy-induced cell death independent of apoptosis. Benzyloxycarbonyl-Val-Ala-Asp zVAD fmk, zVAD was purchased from Calbiochem Mississaga, Ontario and dihydroethidium HE from Invitrogen Burlington, Ontario. HE, 2-ME, zVAD and DAPI were dissolved in dimethyl sulfoxide DMSO. AO, EB and trypan blue were dissolved in 1 × PBS. The final concentration of DMSO in media was less than 0. The concentrations of some reagents used in this study were: H 2 O 2 , 1. Beclin-1 and ATG-5 primary antibodies and their secondary antibody donkey anti-goat HRP were purchased from Santa Cruz Biotechnology, Inc. CA, USA. ATG-7 antibodies were purchased from PromoKine Inc. SOD2 antibodies were purchased from StressGen Biotechnologies Victoria, Canada. Rabbit anti-actin antibody was purchased from Sigma, rabbit anti-LC3 antibody from Abgent Inc. The siRNA specific for human beclin-1 was purchased from Dharmacon Lafayette, CO, USA and the sequences used were same as those by Degenhardt et al. TX, USA targeting exons 1 and 2 and sod-2 siRNA was purchased from Ambion Inc. Medium for the stabilized HeLa cells with overexpression of SOD2 was also supplemented with 0. In this study, except otherwise stated, HeLa cells refer to the wild-type cell line. Cell death was analyzed by measuring the permeability of the plasma membrane to AO-EB or trypan blue. Cell suspension was centrifuged in an eppendorf tube. Live cells are permeable to AO but not to EB and stained green, and dead cells permeable to both AO and EB, and EB stains the DNA red. This red staining is distinctive from AO staining of autolysosomes red in the cytoplasm. At least cells were counted for each treatment. Cell death can also be analyzed by staining cells with trypan blue and counting cells under a microscope. Briefly, cells were collected and suspended in 0. Then, cells were stained with trypan blue with a final concentration of 0. Stained cells were analyzed on a flow cytometer using CellQuest software Becton Dickinson, San Jose, CA. Two peaks in the histograph were observed. The first peak represents viable cells, which were dimly fluorescent and not permeable to trypan blue. The second peak represents dead cells, which were brightly fluorescent and permeable to trypan blue. On the fourth day, cells from each big plate were split into six-well plates with same amount of cells in each well. On the fifth day, old media were removed, and fresh media and H 2 O 2 were added. On the sixth or seventh day, cells were collected and analyzed, and partial cells were lysed to make protein lysates for western blot. Transfection of siRNA into cells follows the Invitrogen protocols with some modifications. The cells were washed once with plain DMEM medium. Then, 2. Autophagy is characterized by the formation of AVOs autophagosomes and autolysosomes. The intensity of the red fluorescence is proportional to the degree of acidity. Thus, the formation of AVOs can be quantified. Cells were washed twice with PBS, resuspended in 0. The DNA was stained with antifade DAPI solution after cells were fixed with 3. Apoptosis was analyzed by measuring sub-G1 peaks indication of DNA fragmentation on a flow cytometer after cells were fixed with ethanol and stained with propidium iodide as stated previously. TUNEL assay Roche Inc. that detects DNA breaks was detected on flow cytometer as per the manufacturer's instructions. ROS generation was determined by flow cytometry after cells were stained with HE. First, HE was dissolved in DMSO to make aliquots of stock solution of 1. When used HE is taken out, covered with aluminum foil, and kept on ice until it is melt. For staining of cells, cells were centrifuged down and the pellet was resuspended in 0. glutaraldehyde in 0. The samples were then washed again, dehydrated with graded alcohol, and embedded in Epon-Araldite resin Canemco Inc. Ultrathin sections were cut on a Reichert ultramicrotome, counterstained with 0. Western blot analysis was performed as stated previously. All experiments were repeated at least three times and each experiment was carried out at least by triplicates. The data were expressed as means±S. Statistical analysis was performed by using Student's t test using at least three independent data points. The software used is excel or sigma blot. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest ; : — Article CAS PubMed PubMed Central Google Scholar. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene ; 23 : — Article CAS PubMed Google Scholar. Mariño G, López-Otín C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. CMLS Cell Mol Life Sci ; 61 : — Article PubMed Google Scholar. Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ ; 12 : — Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol ; 6 : — Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res ; 63 : — CAS PubMed Google Scholar. Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene ; 24 : — Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M et al. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell ; 22 : — Ito H, Aoki H, Kuhnel F, Kondo Y, Kubicka S, Wirth T et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst ; 98 : — Xue L, Fletcher GC, Tolkovsky AM. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr Biol ; 11 : — Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem ; : — Matsui Y, Takagi H, Qu X, Adbellatif M, Sakoda H, Asano T et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res ; : — Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J ; 26 : — Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Update ; 7 : 97— Article CAS Google Scholar. Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA ; : — Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell ; 10 : — Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog ; 5 : Article PubMed PubMed Central Google Scholar. Benassi B, Fanciulli M, Fiorentino F, Porrello A, Chiorino G, Loda M et al. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol Cell ; 21 : — Gao N, Rahmani M, Dent P, Grant S. Takeda M, Shirato I, Kobayashi M, Endou H. Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron ; 81 : — Li L, Heldin NE, Grawe J, Ulmsten U, Fu X. Induction of apoptosis or necrosis in human endometrial carcinoma cells by 2-methoxyestradiol. Anticancer Res ; 24 : — Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie ; 84 : — Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting? Autophagy ; 3 ; e-pub ahead of print. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature ; : — Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol ; 6 : — Buytaert E, Callewaert G, Vandenheede JR, Agostinis P. Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum. Autophagy ; 2 : — Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin Leuk Res ; 31 : — Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell ; 10 : 51— Brunk UT, Dalen H, Roberg K, Hellquist HB. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med ; 23 : — Thorpe GW, Fong CS, Alic N, Higgins VJ, Dawes IW. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol ; 15 : — Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Update ; 6 : — Pribluda VS, Gubish Jr ER, Lavallee TM, Treston A, Swartz GM, Green SJ. Cancer Metast Rev ; 19 : — Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Hagen T, D'Amico G, Quintero M, Palacios-Callender M, Hollis V, Lam F et al. Inhibition of mitochondrial respiration by the anticancer agent 2-methoxyestradiol. Biochem Biophys Res Commun ; : — Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol ; 25 : — Kabore AF, Sun J, Hu X, McCrea K, Johnston JB, Gibson SB. The TRAIL apoptotic pathway mediates proteasome inhibitor induced apoptosis in primary chronic lymphocytic leukemia cells. Apoptosis ; 11 : — Download references. This work was supported from a grant from the Canadian Institutes for Health Research. YC is supported by a post-doctoral fellowship from CancerCare Manitoba Foundation. SBG is supported by a New Investigator Award from the Canadian Institutes for Health Research. We thank Dr. Ludger Klewes for technical support in using the flow cytometer. In addition, special thanks to Elizabeth Henson, Meghan Azad, Xiaojie Hu and WenYan Liu for technical assistance. Manitoba Institute of Cell Biology, McDermot Ave, Winnipeg, Manitoba, Canada. Department of Human Anatomy and Cell Science, Faculty of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada. CancerCare Manitoba, McDermot Ave, Winnipeg, Manitoba, Canada. Biochemistry and Medical Genetics, Faculty of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada. You can also search for this author in PubMed Google Scholar. Correspondence to S B Gibson. Reprints and permissions. Chen, Y. The immunomodulatory effects of MSCs have been extensively studied in the peripheral regions. A variety of cytokines secreted by MSCs have been linked to their immunoregulatory function. These molecules include NO in mice , IDO in human , PGE2, TGF-β, HLA-G5, TSG-6, IL-1Ra, IL, and antagonistic variants of CCL2 Wang et al. The production of these factors inhibits the differentiation, proliferation, and activation of various immune cell subgroups such as macrophages, neutrophils, T cells, B cells, natural killer NK cells, DCs, and mast cells, while it increases the generation of regulatory T cells Treg Li and Hua, Moreover, coculture with MSCs promotes macrophages, DCs, T cells, and NK cells toward anti-inflammatory phenotypes in vitro Cunningham et al. Interestingly, the immunomodulatory properties of MSCs depends on the type and intensities of inflammatory mediators present in the microenvironment. Diverse inflammatory states contribute to dramatically different responses to MSC therapy, suggesting a plasticity of MSCs in immunoregulation Li et al. For instance, MSCs are activated by proinflammatory signals through TLR3 and exert immunosuppressive effects, denoted as MSC2 phenotype, while TLR4 priming results in a proinflammatory phenotype MSC1 accompanied by enhanced T cell responses in the absence of an inflammatory environment Li et al. It is worth mentioning that NO or IDO are key participants and might be the target of manipulating the plasticity of MSC-mediated immunomodulation. In general, the anti-inflammatory molecules generated by MSCs also exhibit immunomodulatory properties in the central nervous system. The immunomodulatory and neuroprotective effects of MSCs in experimental ischemic stroke have also been gradually revealed. These beneficial effects are associated with the modulation of a number of processes, including elevated secretion of anti-inflammatory molecules accompanied by a decline in proinflammatory cytokines, inactivation of inflammatory cells, and inhibition of BBB leakage. Early MSCs administration after stroke notably upregulated the expression level of IL, with decreased TNF-α level in the brain tissue Liu et al. Cheng et al. found that bone marrow mesenchymal stem cells BMSCs attenuated neutrophil infiltration, MMP-9 function, and BBB destruction by inhibiting the expression of intracellular adhesion molecule 1 ICAM-1 in endothelial cells Cheng et al. Another study suggested that MSCs therapy reduced astrocyte apoptosis and inhibited ischemia-induced aquaporin-4 AQP4 upregulation, and this was related to the activation of p38 signaling pathway Tang et al. Meanwhile, that BMSCs inactivated the microglia and induced M2 polarization was further observed by other researchers Li et al. Oh et al. It has been recently shown that employed approaches in vitro were able to enhance the therapeutic potential of MSCs, like molecular priming and tissue engineering Cunningham et al. Many researchers endowed MSCs with more potent anti-inflammatory properties using anti-inflammatory molecules. Other investigators found that CCL2-overexpressing hUMSCs or activation of MSCs with interferon-γ both induced a more forceful anti-inflammatory phenotype of MSCs relative to naive MSCs in ischemic stroke Lee et al. Another significative study showed that the presence of minority population of regulatory T cells in BMSCs conferred them with immunomodulatory and neuroprotection effects against stroke Neal et al. Furthermore, biomaterials have been reported to be able to modulate the behavior of stem cells. Zhai et al. demonstrated that nitrogen-doped carbon nanocages enhanced the therapeutic effects of hUMSCs on cerebral infarction and inhibited the microglia reactivation and neuroinflammation Zhai et al. Besides, Huang and his group coated palmitic acid peptide onto the cell membrane of MSCs and thus increased the number of transplanted cells in the ischemic lesion Huang et al. Generally, these approaches used to modify MSCs might generate a potential therapeutic strategy for stroke management. That MSC-derived EVs can be advantageous over MSCs in the field of stroke therapy is partly dependent on the EV-mediated molecular transfer. EVs always serve as molecular cargoes, such as membrane receptors, proteins, lipids, and various forms of RNA molecules Otero-Ortega et al. Among the contents of EVs, miRNAs, endogenously expressed RNA molecules that function to inhibit messenger RNA mRNA translation, have been shown to govern important processes that are responsible for ischemic stroke injuries Khoshnam et al. Therefore, EV-mediated miRNA transfer provides an attractive candidate for the treatment of cerebral ischemic injury. Recently, a number of studies have confirmed the therapeutic effectiveness of EV-mediated miRNA delivery in ischemic stroke. Plenty of miRNAs were involved in these processes, such as miRNAb-3p and miRNAb-5p Hou et al. However, more researchers used miRNAs to modify MSCs for producing more robust EVs. In their investigations, EVs from MSCs primed with miRNA, miRNAp, miRNAp, miRNAb, and miRNAp showed stronger neuroprotection effects than EVs lacking additional miRNA. Those miRNAs mainly participated in the reduction in neuroinflammation, ROS production, as well as BBB dysfunction, and promotion of angiogenesis Xin et al. Intriguingly, Xin et al. Moreover, other teams designed to modify MSCs in other ways to enhance the therapeutic potential of their EVs. A recent study showed that pretreatment of MSCs with lithium significantly upregulated the expression level of miRNA in MSC-derived EVs, thereby enhancing the resistance of cultured astrocytes, microglia, and neurons against hypoxic injury and reducing the levels of poststroke cerebral inflammation, and this process was connected with miRNA inhibition of TLR4 abundance Haupt et al. Kim et al. There was also a report on the effective inhibition of ROS and inflammatory activity following cerebral ischemia by combined nanoformulation of curcumin and embryonic stem-cell-derived exosomes Kalani et al. Despite that studies on MSC-based therapies that target pyroptosis are relatively few in ischemic stroke, eminent outcome has also been observed. In vitro , the inhibitory effect of BMSC-derived exosomes on pyroptosis in PC12 cells was comparable to the NLRP3 inhibitor and was reversed by NLRP3 overexpression Zeng et al. Meanwhile, that human umbilical cord blood mononuclear cells cbMNCs inhibited the activation of NLRP3 inflammasome in vivo has also been documented Liu et al. Another in vivo investigation demonstrated that lymphocytes cocultured with human cord blood-derived multipotent stem cells HCB-SCs attenuated inflammasome activity in middle cerebral artery occlusion MCAO rats by suppressing NLRP3 inflammasome activation and promoting Tregs differentiation Zhao et al. In addition, a study on microglia revealed that hypoxia-preconditioned OM-MSCs suppressed pyroptotic death of microglia caused by cerebral ischemia—reperfusion insult by activating HIF-1α Huang et al. The mechanism by which MSCs and secretome inhibit pyroptosis has been more deeply studied in other disease models. Several new findings showed that MSCs exosomes inhibited NLRP3 expression and pyroptosis of cardiomyocytes and myocardial infarction by delivering miRNAb or long non-coding RNA lncRNA KLF3-AS1 Mao et al. Liu et al. Besides, Kong and his group transplanted IL gene-modified MSCs into rats model of intestinal ischemia—reperfusion injury and found that the expression of NLRP3 and downstream targets cleaved caspase-1, IL-1β, and IL were observably lessened Kong et al. Overall, the underlying mechanism regarding multiple molecular pathways involved in the role of MSCs and secretome in other diseases are expected to further elucidate in ischemic stroke. Different from the direct inhibition of oxidative stress level and inflammatory activity, MSCs have two-sided effects on autophagy in ischemic stroke. Accumulating evidence have implied that MSCs were able to suppress autophagy through numerous molecular pathways and then promoted functional recovery after ischemic injury. Among these researches, Li et al. Second, exosome-mediated miRNAs delivery also took part in the regulatory process. miRNAa in exosomes from hUMSCs directly binds to beclin-1 and inhibits its expression, thereby inhibiting autophagic flux in ischemia—reperfusion-induced injury Zhang et al. Third, a recent study suggested that the protective role of transplanted MSCs in a murine model of ischemic stroke was associated with their promotion of the molecular switch from autophagy to ubiquitin—proteasome system UPS Tadokoro et al. By contrast, some other investigations declared that MSCs combated ischemic injury by enhancing autophagy Huang et al. Likewise, in most studies, MSCs play a role by targeting mTOR-mediated autophagy pathway. In PC12 cells treated with OGD insult, BMSC exosomes attenuated the pyroptosis mediated by NLRP3 inflammasome by promoting AMPK-dependent autophagy flux Zeng et al. Besides, heme oxygenase-1 HO-1 -mediated autophagy could also be modulated by MSCs in ischemic injury models Wang et al. Collectively, the regulatory role of MSCs in autophagy following ischemic stroke is still under dispute. Even in the same cell or animal models of cerebral ischemic injury, MSCs can exhibit diametrically opposite effects on the modulation of autophagy, which is believed to be related to multiple factors, such as the length of modeling time and the time nodes of MSCs intervention. From another point of view, the beneficial or detrimental impacts on ischemic brain tissue depend on the intensity of autophagy, and the transplanted MSCs exert neuroprotection effects through modulating their functions adaptively according to the state of autophagy. The applicable therapeutic strategy to reduce or prevent the cerebral ischemic injury is still largely lacking. Abundant data implicated intricate rather than a single signaling pathway to frequently work together to undermine the cells in the setting of cerebral ischemia—reperfusion. The crosstalk among oxidative stress, inflammatory activity, and autophagy dysfunction may raise the need of deeply taking into consideration these pathways in ischemic stroke. Nowadays, the pleiotropic ability of MSCs to exhibit antioxidative stress, reduce neuroinflammation, and regulate autophagy in experimental ischemic stroke has been recognized, most of which benefit from its robust paracrine activities Figure 2. More importantly, the low immunogenicity, ability to cross the BBB, capacity of targeted delivering gene drugs, and similar properties as MSCs seem to make MSC-derived EVs a better clinical application candidate relative to MSCs. In summary, MSCs and secretome hold great promise in the clinical treatment of ischemic stroke. Figure 2. MSCs rescue ischemic brain tissue and promote recovery by inhibiting oxidative stress as well as inflammatory activity and modulation autophagy. MSCs, mesenchymal stem cells; TNTs, tunneling nanotubes; EVs, extracellular vesicles; ROS, reactive oxygen species; RNS, reactive nitrogen species; BBB, blood—brain barrier; UPS, ubiquitin-proteasome system; HO-1, heme oxygenase ZH and HX acquired the funding. JH attended in literature review and drafting the manuscript. JL and YH participated in literature review. XT and ZH supervised the project. All authors read and approved the final manuscript. This work was supported by the National Natural Science Foundation of China grant numbers and and the Natural Science Foundation of Hunan Province, China grant number JJ The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Acquistapace, A. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 29, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Albers, G. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. Alhazzani, A. Cells Allen, C. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Stroke 4, — Anderson, C. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke ENCHANTED : an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet , — CrossRef Full Text Google Scholar. Anrather, J. Inflammation and stroke: an overview. Neurotherapeutics 13, — Babenko, V. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells MMSC to neural cells and improves the efficacy of cell recovery. Molecules Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: the role of crosstalk between cells. Stem Cells Transl. Bao, Q. Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano 12, — Bergendi, L. Chemistry, physiology and pathology of free radicals. Life Sci. Bernardo, M. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, — Boltze, J. Stem cells as an emerging paradigm in stroke 4: advancing and accelerating preclinical research. Stroke 50, — Boshuizen, M. Stem cell—based immunomodulation after stroke. Stroke 49, — Calio, M. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol. Campbell, B. Ischaemic stroke. Primers Carbone, F. Pathophysiology and treatments of oxidative injury in ischemic stroke: focus on the phagocytic NADPH Oxidase 2. Castets, P. Cecconi, F. The role of autophagy in mammalian development: cell makeover rather than cell death. Cell 15, — Cespedes, A. Energy-Sensing pathways in ischemia: the counterbalance between AMPK and mTORC. Chamorro, Á, Dirnagl, U. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. Chen, H. Chen, S. Targeting Myeloperoxidase MPO mediated oxidative stress and inflammation for reducing brain ischemia injury: potential application of natural compounds. Acta Pharmacol. Chen, K. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells ADMSC and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7, — Chen, X. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Cheng, Z. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. Chong, Z. The rationale of targeting mammalian target of rapamycin for ischemic stroke. Cell Signal. Cunningham, C. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. Blood Flow Metab. Systemic conditioned medium treatment from interleukin-1 primed mesenchymal stem cells promotes recovery after stroke. Stem Cell Res. Darroudi, S. Biofactors 46, 55— Deans, R. Mesenchymal stem cells: biology and potential clinical uses. Deng, Y. Exosomes derived from microRNAp-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. Elsner, V. Therapeutic effectiveness of a single exercise session combined with WalkAide functional electrical stimulation in post-stroke patients: a crossover design study. Feng, J. Reactive nitrogen species as therapeutic targets for autophagy: implication for ischemic stroke. Expert Opin. Targets 21, — Ferreira, J. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Fu, B. Cell Longev. Furuhashi, M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. GBD Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for diseases and injuries for countries and territories, a systematic analysis for the Global Burden of Disease Study Gelderblom, M. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, — George, P. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87, — Gong, X. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. Cell Physiol. Granger, D. Reperfusion injury and reactive oxygen species: the evolution of a concept. Guan, R. Mitophagy, a potential therapeutic target for stroke. Guan, Y. Stem Cells Int. Guo, Q. ATM-CHK2-Beclin 1 axis promotes autophagy to maintain ROS homeostasis under oxidative stress. EMBO J. Guo, Z. Keap1-Nrf2 signaling pathway in angiogenesis and vascular diseases. Tissue Eng. Guruswamy, R. Complex roles of microglial cells in ischemic stroke pathobiology: new insights and future directions. Haupt, M. Lithium modulates miR levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Cells Transl. CrossRef Full Text PubMed Abstract Google Scholar. He, H. Brain Res. He, J. Cell Dev. Hendouei, N. Alterations in oxidative stress markers and its correlation with clinical findings in schizophrenic patients consuming perphenazine, clozapine and risperidone. Hong, Y. High-frequency repetitive transcranial magnetic stimulation improves functional recovery by inhibiting neurotoxic polarization of astrocytes in ischemic rats. Hou, K. Bone mesenchymal stem cell-derived exosomal microRNAb-3p prevents hypoxic-ischemic injury in rat brain by activating the PTEN-mediated Akt signaling pathway. The progress of neuronal autophagy in cerebral ischemia stroke: mechanisms, roles and research methods. Hu, Z. Mechanism and regulation of autophagy and its role in neuronal diseases. Huang, B. Peptide modified mesenchymal stem cells as targeting delivery system transfected with miRb for the treatment of cerebral ischemia. Huang, X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Cell Res. Huang, Y. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics 8, — Aging Albany NY 12, — Iadecola, C. The immunology of stroke: from mechanisms to translation. Jayaraj, R. Neuroinflammation: friend and foe for ischemic stroke. Jiang, Q. Hypoxia Inducible Factor-1alpha HIF-1alpha mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience , — Jiang, T. Ischemic preconditioning provides neuroprotection by induction of AMP-activated protein kinase-dependent autophagy in a rat model of ischemic stroke. Jiang, Z. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Jorgensen, I. Pyroptotic cell death defends against intracellular pathogens. Kalani, A. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Cell Biol. Kanazawa, M. Kang, J. Mitochondria: redox metabolism and dysfunction. Khoshnam, S. Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. Stroke 19, — Kim, H. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials Kim, K. Role of autophagy in endothelial damage and blood—brain barrier disruption in ischemic stroke. Koike, M. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Kong, D. IL Gene modification enhances the protective effects of mesenchymal stromal cells on intestinal ischemia reperfusion injury. Kotur-Stevuljevic, J. Oxidative stress and paraoxonase 1 status in acute ischemic stroke patients. Atherosclerosis , — Kuang, Y. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR Vesicles 10, e The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. Lee, J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Lee, S. Enhancing the therapeutic potential of CCL2-Overexpressing mesenchymal stem cells in acute stroke. Leu, S. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. Li, D. Autologous transplantation of adipose-derived mesenchymal stem cells attenuates cerebral ischemia and reperfusion injury through suppressing apoptosis and inducible nitric oxide synthase. Li, W. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. Li, F. Li, X. Li, Y. Li, Z. Bone marrow-mesenchymal stem cells modulate microglial activation in the peri-infarct area in rats during the acute phase of stroke. Li, G. Cell Cycle 19, — Li, J. Mesenchymal stem cell therapy for ischemic stroke: a look into treatment mechanism and therapeutic potential. Li, T. Effects of AMP-activated protein kinase in cerebral ischemia. Flow Metab. Cell Mol. Li, L. ROS and autophagy: interactions and molecular regulatory mechanisms. Li, N. Interactions between mesenchymal stem cells and the immune system. Liddelow, S. Neurotoxic reactive astrocytes are induced by activated microglia. Nature , — Liesz, A. DAMP signaling is a key pathway inducing immune modulation after brain injury. Lin, K. Xenogeneic and allogeneic mesenchymal stem cells effectively protect the lung against ischemia-reperfusion injury through downregulating the inflammatory, oxidative stress, and autophagic signaling pathways in rat. Cell Transplant Liu, H. Cell Biochem. Liu, Y. Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Autophagy 15, — Liu, J. Crosstalk between autophagy and ferroptosis and its putative role in ischemic stroke. Cell Neurosci. Chitosan hydrogel enhances the therapeutic efficacy of bone marrow—derived mesenchymal stem cells for myocardial infarction by alleviating vascular endothelial cell pyroptosis. Liu, L. Transplantation of human umbilical cord blood mononuclear cells attenuated ischemic injury in MCAO rats via inhibition of NF-κB and NLRP3 inflammasome. Liu, N. Expression of IL and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Liu, W. |
0 thoughts on “Autophagy and oxidative stress”