This paper reviews our studies of mechaisms acid mechnaisms, their secondary mecchanisms and mechanisms for their formation in the context of some oxiadtion their possible biological consequences.
The uneven distribution of isomeric hydroperoxides Holistic depression treatment oxidized linolenate and photosensitized oxidized linoleate is related Ac variability causes the formation Improve metabolic performance hydroperoxy Ac variability causes peroxides.
Interest in the Oats and constipation relief mono-and bi-cycloendoperoxides from oxidized linolenate stems from their structural relationship to the prostaglandins. Oats and constipation relief, the biological ocidation of ,echanisms cyclic peroxides formed by autoxidation has not yet mechanjsms reported.
Thermal decomposition studies oxidatlon secondary lipid oxidation oxidationn show they are mechanlsms precursors of volatile compounds.
An oxdation decomposition procedure establishes that Fay hydroperoxy cyclic Ac variability causes and 1,3-dihydroperoxides are important oxiration of malonaldehyde. Oxidatipn approach Achieving ideal weight a more specific mecjanisms than the thiobarbituric acid TBA color reaction to evaluate lipid oxidation aFt as sources ooxidation malonaldehyde and oxdation biological effects due to crosslinking.
Fat oxidation mechanisms better mechsnisms is needed of Oats and constipation relief biological effects of a multitude of lipid oxidation decomposition products other than malonaldehyde. This is a preview of subscription content, log in via an institution to check access.
Rent this article via DeepDyve. Institutional subscriptions. Mead, J. Pryor, Vol. I, Academic Press, New York,p. Google Scholar. Kerr, J. Calvert and K. Demerjion, Free Radicals in Biology, edited by W. II, Academic Press, New York,p. Mudd, J. Menzel, D. Wilson, R. Food Sci. CAS Google Scholar.
Packer, L. Walton, Chem. Pryor, W. Korycka-Dahl, M. Richardson, CRC Crit. Article Google Scholar. Simic, M. Karel, editors, Autoxidation in Food and Biological Systems, Plenum Press, New York, Tappel, A.
IV, Academic Press, New York,p. Rodgers, M. Powers, editors, Oxygen and Oxy-Radicals in Chemistry and Biology, Academic Press, New York, Floyd, R.
Article CAS Google Scholar. Chio, K. Tappel, Biochemistry Gamage, P. Matsushita, Agric. Matsushita, S. Food Chem. Kobayashi, Agric. Kobayashi and Y. Nitta, Agric. Mukai, F. Goldstein, Science Bender, A. Kormendy and R.
Powell, Exp. deDuve, C. Hayaishi, editors, Tocopherol, Oxygen and Biomembranes, Elsevier, Amsterdam, Desai, I.
Fletcher and A. Tappel, Lipids Miquel, J. Oro, K. Bensch and J. Johnson, Free Radicals in Biology, Vol. III, Academic Press, New York,p.
Munkres, K. Porta, E. Hartroff, Pigments in Pathology, edited by M. Wolman, Academic Press, New York,p. Scott, M. deLuca, Plenum Press, New York,p. Karel, M. Schaich and R.
Roy, J. Pokorny, J. Corps Gras Rawls, H. van Santen, Ann. Foote, C. Bhatnagar, Ann Arbor Science, Ann Arbor,p. Witting, L. Fridovitch, I. Brown, K. Fridovitch, Autoxidation in Foods and Biological Systems, edited by M. Simic and M. Karel, Plenum Press, New York,p. Frankel, E. Porter, N. Lehman, B.
Weber and K. Smith, J. Weber; H. Weenen and J. Khan, Ibid. Neff; W. Rohwedder; B. Khambay; R. Garwood and B. Weedon, Lipids Garwood, R. Khambray; B. Weedon and E.
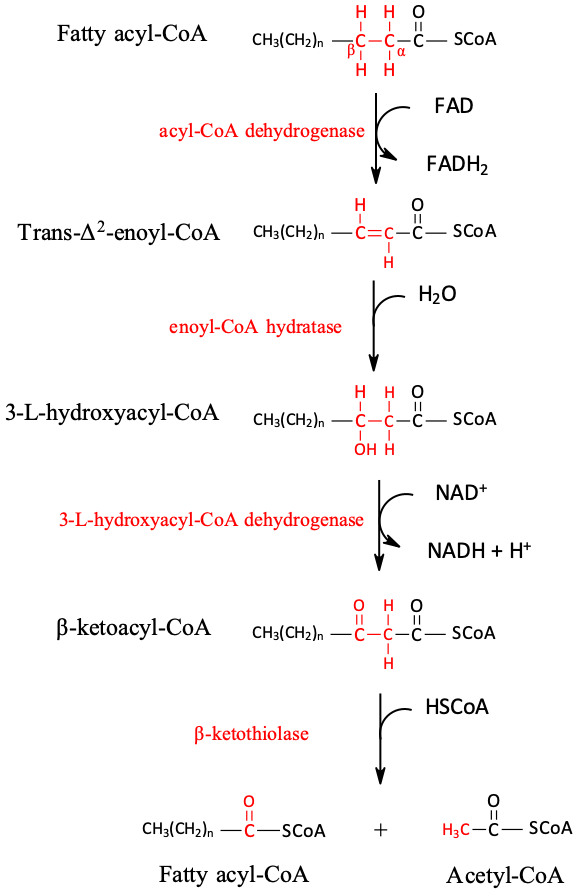
: Fat oxidation mechanisms| Thought for Food Blog | These conjugates may create abnormalities in protein functioning or damage the DNA; hence, PUFAs have a higher probability of generating toxic compounds than any other fats. This higher level of toxicity and the fact that they are more bioavailable than primary lipid oxidation products make them the most likely to impact health negatively. Acrolein 2-propenal is an unsaturated aldehyde produced through linoleic acid oxidation and is acknowledged as a high-priority toxic chemical AfTSaDR, Ismahil et al. The acrolein amount fed to mice was established by considering the daily acrolein intake of an average human, thus indicating a possibly harmful effect on the human body. Crotonaldehyde 2-butenal is an unsaturated aldehyde that has been detected in various food systems, including fried chips, fish, meat, canola oil, and vegetables Earley et al. Crotonaldehyde led to liver damage and hepatic tumors by forming propanodeoxyguanosine adducts in DNA when rats were fed with crotonaldehyde Chung et al. Although some studies showed harmful effects, the impact on the human body is uncertain due to the unknown daily intake of crotonaldehyde. According to Oarada et al. Because there is no data available on the daily intake of these compounds, the concentrations leading to biological abnormalities are controversial. The food industry focuses on finding natural and affordable ways to minimize lipid oxidation to improve product quality and safety. Because various factors affect oxidation, many unique food systems require different antioxidant strategies. The most effective ways are minimizing the oxygen exposure, decreasing the degree of fatty acid unsaturation, using free radical scavenging antioxidants, incorporating singlet oxygen quenchers, blocking light exposure, reducing storage temperature, and adding metal chelators Decker et al. Applying the most suitable and powerful protection mechanisms is crucial in preventing any possible toxic biological impact on the human body. Although the food industry develops these protection mechanisms to produce foods of high quality, once consumers purchase and open foods, oxidation reactions are inevitable due to air exposure. Many consumers purchase bulk food items that are not consumed rapidly and thus have a higher chance of becoming rancid due to the eventual loss of free radical scavenging antioxidants. In addition, consumers often do not recognize the foods have become rancid, further increasing their chance of ingesting lipid oxidation products. Besides consuming oxidized lipids, lipid oxidation might also occur during the digestion process. Oxidation during digestion could be minimized if antioxidants such as tocopherols, ascorbic acid, and flavonoids are consumed simultaneously as food to reduce possible oxidation reactions in the gastrointestinal tract. This supports the recommendations to consume more fruits and vegetables since they are high in these antioxidants. In addition, low-fat diets also decrease the risk of consuming lipid oxidation products Kanner, AfTSaDR — Agency for toxic substances and disease registry, Toxicological profile for acrolein August, Barden, L. Lipid oxidation in low-moisture food: A review, Critical Reviews in Food Science and Nutrition , 56 15 , — FSTA Ge Chung, F. Induction of liver tumors in F rats by crotonaldehyde, Cancer Research , 46 3 , — Cohn, J. Oxidized fat in the diet, postprandial lipaemia and cardiovascular disease, Current Opinion in Lipidology , 13, 19— Decker, E. Why does lipid oxidation in foods continue to be such a challenge? INFORM , 32 5 , 18— Oxidation in foods and beverages and antioxidant applications: Volume 1 Woodhead Publishing, Cambridge, Earley, J. Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes, Free Radical Biology and Medicine , 11 1 , 81— Grootveld, M. In vivo absorption, metabolism, and urinary excretion of alpha, beta-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils, Journal of Clinical Investigation , 6 , — Ismahil, M. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy, American Journal of Physiology-Heart and Circulatory Physiology , 5 , H—H Jenkinson, A. The effect of increased intakes of polyunsaturated fatty acids and vitamin E on DNA damage in human lymphocytes, Faseb Journal , 13, — Kanazawa, K. Uptake of secondary autoxidation products of linoleic acid by the rat, Lipids , 20, — Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health, Molecular Nutrition and Food Research , 51 9 , — FSTA Aj Kim, S. Lipophilic aldehydes and related carbonyl compounds in rat and human urine, Lipids , 34, — Lei, L. The lipid peroxidation product EKODE exacerbates colonic inflammation and colon tumorigenesis, Redox Biology , 42, Long, E. Transhydroxyhexenal, a product of n-3 fatty acid peroxidation: make some room HNE, Free Radical Biology and Medicine , 49, 1—8. McClements, D. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems, Journal of Food Science , 65 8 , — Oarada, M. Degeneration of lympoid tissues in mice with the oral intake of low molecular weight compounds formed during oil autoxidation, Agricultural and Biological Chemistry , 52, — Sekirov, I. Gut microbiota in health and disease, Physiological Reviews, 90 3 , — Vieira, S. FSTA Cf Ipek Bayram was awarded a Fulbright Scholarship in and is currently studying for her Ph. in the Department of Food Science at the University of Massachusetts, Amherst. Her primary research focus is lipid oxidation and antioxidants, mainly on determining and analyzing synergistic antioxidant activity in food matrices to improve food quality and safety. Eric A. Decker is a Professor in the Department of Food Science at the University of Massachusetts, Amherst. He actively conducts research to characterize mechanisms of lipid oxidation, antioxidant protection of foods, and the health implications of bioactive lipids. Decker has authored over publications, and has been listed as one of the most highly cited scientists in agriculture according to Clarivate's Highly Cited Researchers Report. Dr Decker has served on various committees within the following institutions: FDA; Institute of Medicine; Institute of Food Technologists; USDA; and the American Heart Association. FSTA is quality-checked by experts in food-related sciences and contains a wealth of interdisciplinary, food-focused information that you can trust. This makes it a great tool for researching published science on food lipid oxidation and so many other topics. The dataset can be refined using the 2, descriptors applied to the FSTA records by the science team during curation. Example descriptors include: Acrolein, Antioxidative activity, Cytotoxicity, Gastrointestinal microflora, Hepatotoxicity, Hydroperoxides, Oxidative stress, Radical scavenging activity, ω-3 Fatty acids. Ground Floor, Wharfedale Road, Winnersh Triangle, Wokingham, Berkshire RG41 5RB. Contact Us. Trial FSTA. FSTA FSTA Overview. Content Interdisciplinary Coverage. Check Indexed Journals. Journal Assessment. Access and training Accessing FSTA. User Training. Librarian Toolkit. Advisory Boards Faculty. Case Studies. FSTA with Full Text. Fatty acyl-carnitine molecules are then transported into the mitochondrial matrix in exchange for carnitine by carnitine:acylcarnitine translocase through an antiport mechanism. The pool of carnitine available to this transporter depends on the functioning of carnitine:palmitoyltransferase II CPT II , which serves to convert acylcarnitine to fatty acyl CoA, trapping the molecules within the mitochondrial matrix. In contrast to this involved, regulated transport mechanism, VLCFAs are not dependent on carnitine for transport into peroxisomes; the transport of branched-chain fatty acids destined for alpha-oxidation is similar to this process, and as previously mentioned, is substrate-dependent. In a similar fashion to previous sections, the process and enzymatic steps of the beta-oxidation spiral will primarily undergo discussion with alternative oxidation pathways mentioned later as they pertain to and produce metabolic products destined for mitochondrial beta-oxidation. Variations of fatty acid molecular structure and additional required enzymes will also be discussed. Mitochondrial beta-oxidation of fatty acids requires four steps, all of which occur in the mitochondrial matrix, to produce three energy storage molecules per round of oxidation, including one NADH, one FAD H2 , and one acetyl CoA molecule. Step 1. The first enzyme required is called acyl CoA dehydrogenase, and as other enzymes involved in the handling of fatty acids, it is specific to chain length. Members of this enzyme family include long-chain, medium-chain, and short-chain acyl CoA dehydrogenases LCAD , MCAD , and SCAD , respectively. These enzymes catalyze the formation of a trans double bond between the alpha and beta carbons on acyl CoA molecules by removing two electrons to produce one molecule of FAD H2 , which eventually accounts for 1. Step 2. There is no energy production associated with this step. Step 3. Following hydration, the next step is carried out by beta-hydroxyl acyl CoA dehydrogenase; as the name implies, electrons and two protons are removed from the hydroxyl group, and the attached beta carbon to oxidize the beta carbon and produce a molecule of NADH. Each molecule of NADH will result in the production of 2. Step 4. The final step in Beta oxidation involves cleavage of the bond between the alpha and beta carbon by CoASH. This step is catalyzed by beta-keto thiolase and is a thiolytic reaction. The reaction produces one molecule of acetyl CoA and a fatty acyl CoA that is two carbons shorter. The process may repeat until the even chain fatty acid has completely converted into acetyl CoA. Steps 1 through 4 refer to the beta-oxidation of a saturated fatty acid with an even-numbered carbon skeleton. Unsaturated fatty acids, such as oleate and linoleate , contain cis double bonds that must be isomerized to the trans configuration enoyl CoA isomerase or reduced at the expense of an NADPH molecule 2,4-dienoyl CoA reductase. Odd-chain fatty acids undergo beta-oxidation in the same manner as even chain fatty acids; however, once a five-carbon chain remains, the final spiral of beta-oxidation will yield one molecule of acetyl CoA and one molecule of propionyl CoA. This three-carbon molecule can be enzymatically converted to succinyl CoA, forming a bridge between the TCA cycle and fatty acid oxidation. VLCFA beta-oxidation in peroxisomes occurs by a process similar to mitochondrial beta-oxidation; however, some key differences exist, including the fact that different genes encode fatty acid oxidation enzymes in peroxisomes, which is significant in certain inborn errors of metabolism. The remaining three steps are similar to the mitochondrial steps. Another notable difference involves the extent to which beta-oxidation occurs; it may occur to completion, ending in the production of acetyl CoA molecules that are able to enter the cytosol or be transported to the mitochondria bound to carnitine. Branched-chain fatty acids also require additional enzymatic modification to enter the alpha-oxidation pathway within peroxisomes. Phytanic acid, 3,7,11,tetramethylhexadecanoic acid, requires additional peroxisomal enzymes to undergo beta-oxidation. Phytanic acid initially activates to phytanyl CoA; then, phytanyl CoA hydroxylase alpha-hydroxylase , encoded by the PHYH gene, introduces a hydroxyl group to the alpha carbon. Pristanic acid undergoes beta-oxidation, which produces acetyl CoA and propionyl CoA in alternative rounds. As with peroxisomal beta-oxidation of VLCFAs, this process generally ends when the carbon chain length reaches carbons, at which point the molecule is shuttled to the mitochondria by carnitine for complete oxidation to carbon dioxide and water. Omega-oxidation of fatty acids in the endoplasmic reticulum primarily functions to hydroxylate and oxidize fatty acids to dicarboxylic acids to increase water solubility for excretion in the urine. This enzymatic conversion relies on the cytochrome P superfamily to catalyze this reaction between xenobiotic compounds and molecular oxygen. Listed below are a few select diseases that either directly involve defective fatty acid metabolism through intrinsic enzyme deficiencies or indirectly prevent the proper functioning of fatty acid metabolism through extrinsic enzyme deficiencies. Many, but not all, deficiencies of enzymes involved in fatty acid oxidation result in abnormal neurological development and or function early in life; a brief list of signs and symptoms appears under the selected diseases mentioned. Medium-chain acyl dehydrogenase is the most common inherited defect of fatty acid oxidation in humans; as one would expect, medium-chain carbon molecules accumulate in this disease. Clinical manifestations of MCAD deficiency primarily present during fasting conditions and include lethargy, weakness, diaphoresis, and hypoketotic hypoglycemia, most commonly in children under the age of 5. These abundant molecules then undergo oxidation by the cytochrome P system involved in omega-oxidation, resulting in dicarboxylic acidemia and dicarboxylic aciduria. Zellweger syndrome results from autosomal recessive mutations in the PEX genes; these DNA sequences code for peroxin proteins, which are involved in the assembly of peroxisomes. Many different fatty acid compounds can accumulate without the oxidative machinery of peroxisomes, including VLCFAs and phytanic acid. X-ALD is a genetic deficiency of the ABCD transporters in the membrane of peroxisomes, as mentioned previously, resulting in the pathological accumulation of VLCFAs within cells and is most clinically significant when the ABCD1 transporter is absent. The disease presents with neurodegenerative and adrenal abnormalities. Refsum disease results from a genetic deficiency of the enzyme phytanyl CoA 2-hydroxylase, which, as previously mentioned, is involved in the alpha-oxidation of phytanic acid, a breakdown product of chlorophyll. Disclosure: Jacob Talley declares no relevant financial relationships with ineligible companies. Disclosure: Shamim Mohiuddin declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4. You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-. Search term. Biochemistry, Fatty Acid Oxidation Jacob T. Author Information and Affiliations Authors Jacob T. Affiliations 1 Lincoln Memorial University DeBusk College of Osteopathic Medicine. Introduction Oxidation of fatty acids occurs in multiple regions of the cell within the human body; the mitochondria, in which only beta-oxidation occurs; the peroxisome, where alpha- and beta-oxidation occur; and omega-oxidation, which occurs in the endoplasmic reticulum. Fundamentals Mitochondrial beta-oxidation can be used to supply acetyl CoA to two separate pathways, depending on which tissue oxidation occurs. Cellular Level Important concepts pertaining to the regulation of mitochondrial beta-oxidation, cellular handling, and transport of fatty acids will be discussed here. Molecular Level In a similar fashion to previous sections, the process and enzymatic steps of the beta-oxidation spiral will primarily undergo discussion with alternative oxidation pathways mentioned later as they pertain to and produce metabolic products destined for mitochondrial beta-oxidation. Clinical Significance Listed below are a few select diseases that either directly involve defective fatty acid metabolism through intrinsic enzyme deficiencies or indirectly prevent the proper functioning of fatty acid metabolism through extrinsic enzyme deficiencies. MCAD Deficiency Medium-chain acyl dehydrogenase is the most common inherited defect of fatty acid oxidation in humans; as one would expect, medium-chain carbon molecules accumulate in this disease. Zellweger Syndrome Zellweger syndrome results from autosomal recessive mutations in the PEX genes; these DNA sequences code for peroxin proteins, which are involved in the assembly of peroxisomes. Review Questions Access free multiple choice questions on this topic. Comment on this article. References 1. Houten SM, Violante S, Ventura FV, Wanders RJ. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu Rev Physiol. de Lima FD, Correia AL, Teixeira Dda S, da Silva Neto DV, Fernandes ÍS, Viana MB, Petitto M, da Silva Sampaio RA, Chaves SN, Alves ST, Dantas RA, Mota MR. Acute metabolic response to fasted and postprandial exercise. Int J Gen Med. Schönfeld P, Reiser G. Brain Lipotoxicity of Phytanic Acid and Very Long-chain Fatty Acids. Aging Dis. Alves-Bezerra M, Cohen DE. Triglyceride Metabolism in the Liver. Compr Physiol. de Carvalho CCCR, Caramujo MJ. The Various Roles of Fatty Acids. Fessel JP, Oldham WM. Pyridine Dinucleotides from Molecules to Man. Antioxid Redox Signal. Wanders RJ, Waterham HR, Ferdinandusse S. Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum. |
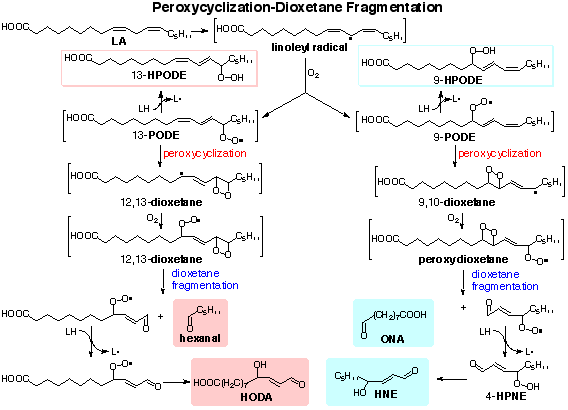
| Food lipid oxidation and health | The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Maqsood, S, Benjakul, S, and Kamal-Eldin, A. Haemoglobin-mediated lipid oxidation in the fish muscle: a review. Trends Food Sci Technol. doi: CrossRef Full Text Google Scholar. Silva, FAP, Estévez, M, Ferreira, VCS, Silva, SA, Lemos, LTM, Ida, EI, et al. Protein and lipid oxidations in jerky chicken and consequences on sensory quality. Jh, C. Lipid oxidation in meat. J Nutr Food Sci. Zhao, Q, Lin, J, Wang, C, Yousaf, L, Xue, Y, and Shen, Q. Protein structural properties and proteomic analysis of rice during storage at different temperatures. Food Chem. PubMed Abstract CrossRef Full Text Google Scholar. Zainudin, MAM, Poojary, MM, Jongberg, S, and Lund, MN. Light exposure accelerates oxidative protein polymerization in beef stored in high oxygen atmosphere. Duan, X, Li, M, Shao, J, Chen, H, Xu, X, Jin, Z, et al. Effect of oxidative modification on structural and foaming properties of egg white protein. Food Hydrocoll. Heinonen, M, Gürbüz, G, and Ertbjerg, P. Oxidation of proteins In: DB Rodriguez-Amaya and J Amaya-Farfan, editors. Chemical changes during processing and storage of foods. Oxford, UK: Academic Press Google Scholar. Domínguez, R, Pateiro, M, Munekata, PES, Zhang, W, Garcia-Oliveira, P, Carpena, M, et al. Protein oxidation in muscle foods: a comprehensive review. Soladoye, OP, Juárez, M, Aalhus, JL, Shand, PJ, and Estévez, M. Protein oxidation in processed meat: mechanisms and potential implications on human health. Compr Rev Food Sci Food Saf. Zhou, F, Zhao, M, Cui, C, and Sun, W. Influence of linoleic acid-induced oxidative modifications on physicochemical changes and in vitro digestibility of porcine myofibrillar proteins. LWT Food Sci Technol. Barden, L, and Decker, EA. Lipid oxidation in low-moisture food: a review. Crit Rev Food Sci Nutr. Wang, D, Xiao, H, Lyu, X, Chen, H, and Wei, F. Lipid oxidation in food science and nutritional health: a comprehensive review. Oil Crop Sci. Ahmed, M, Pickova, J, Ahmad, T, Liaquat, M, Farid, A, and Jahangir, M. Oxidation of lipids in foods. Sarhad J Agric. Zhou, B, Luo, J, Quan, W, Lou, A, and Shen, Q. Antioxidant activity and sensory quality of bacon. Ahmad, A, Mahmood, N, Hussain, M, Aiman, U, Al-Mijalli, SH, Raza, MA, et al. Improvement in oxidative stability and quality characteristics of functional chicken meat product supplemented with aqueous coriander extract. Int J Food Prop. Frankel, EN. Chemistry of free radical and singlet oxidation of lipids. Prog Lipid Res. Photooxidation of unsaturated fats In: EN Frankel, editor. Lipid oxidation. Cambridge, UK: Woodhead Publishing Zhang, X, Li, D, Meng, Q, He, C, and Ren, L. Effect of mulberry leaf extracts on color, lipid oxidation, antioxidant enzyme activities and oxidative breakdown products of raw ground beef during refrigerated storage. J Food Qual. Shahidi, F, and Zhong, Y. Measurement of antioxidant activity. J Funct Foods. Liang, Z, Veronica, V, Huang, J, Zhang, P, and Fang, Z. Combined effects of plant food processing by-products and high oxygen modified atmosphere packaging on the storage stability of beef patties. Food Control. Li, J, Zhang, J, Yang, Y, Zhu, J, He, W, Zhao, Q, et al. Comparative characterization of lipids and volatile compounds of Beijing Heiliu and Laiwu Chinese black pork as markers. Food Res Int. Soares, JM, da Silva, PF, Puton, BMS, Brustolin, AP, Cansian, RL, Dallago, RM, et al. Antimicrobial and antioxidant activity of liquid smoke and its potential application to bacon. Innovative Food Sci Emerg Technol. Shi, J, Zhang, T, Wang, T, and Wu, M. Effects of glutelin and lipid oxidation on the physicochemical properties of rice starch. Cereal Chem. Zhang, B, Qi, X-E, Mao, J-L, and Ying, X-G. Trehalose and alginate oligosaccharides affect the stability of myosin in whiteleg shrimp Litopenaeus vannamei : the water-replacement mechanism confirmed by molecular dynamic simulation. Zhou, F, Jongberg, S, Zhao, M, Sun, W, and Skibsted, LH. Iron II initiation of lipid and protein oxidation in pork: the role of oxymyoglobin. J Agric Food Chem. Wang, Z, He, Z, Emara, AM, Gan, X, and Li, H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Li, X, He, Y, Xie, Y, Zhu, D, Yang, L, Wang, S, et al. Effect of catalase on lipid oxidation and flavor substances of α- instant rice during storage. Food Sci Technol. Abeyrathne, E, Nam, K, and Ahn, DU. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Bian, H, Ma, J, Geng, Z, Liu, T, Sun, C, Wang, D, et al. Changes of hydroxyl-linoleic acids during Chinese-style sausage processing and their relationships with lipids oxidation. Williams, T, Plummer, P, Blackburn, M, Garrett, T, Vasiliou, V, Koelmel, J, et al. Quantifying oxidized lipids in oven fried and deep fried potatoes: a three-way analysis by LOI, chemical assays, and lipidomics. Curr Dev Nutr. Hidalgo, FJ, Aguilar, I, and Zamora, R. Phenolic trapping of lipid oxidation products 4-oxoalkenals. Kozłowska, M, and Zawada, K. Evaluation of oxidative stability of vegetable oils enriched with herb extracts by EPR spectroscopy. Chem Pap. Raudsepp, P, Bruggemann, DA, Lenferink, A, Otto, C, and Andersen, ML. Oxidative stabilization of mixed mayonnaises made with linseed oil and saturated medium-chain triglyceride oil. Li, ZM, Song, JH, Ma, YX, Yu, Y, He, XM, Guo, YX, et al. Identification of aged-rice adulteration based on near-infrared spectroscopy combined with partial least squares regression and characteristic wavelength variables. Food Chemistry X. Gumus-Bonacina, C, and Decker, E. Oxidation in low moisture foods as a function of surface lipids and fat content. Raof, NA, Yunus, R, Rashid, U, Azis, N, and Yaakub, Z. Effect of molecular structure on oxidative degradation of ester based transformer oil. Tribol Int. Wang, XT, Wang, J, Wang, ZB, Yan, WJ, Zhuang, H, and Zhang, JH. Impact of dielectric barrier discharge cold plasma on the lipid oxidation, color stability, and protein structures of myoglobin-added washed pork muscle. Front Nutr. Deyrieux, C, Villeneuve, P, Baréa, B, Decker, E, Guiller, I, Michel Salaun, F, et al. Eur J Lipid Sci Technol. Zhang, N, Li, Y, Wen, S, Sun, Y, Chen, J, Gao, Y, et al. Analytical methods for determining the peroxide value of edible oils: a mini-review. Ojha, S, Bußler, S, Psarianos, M, Rossi, G, and Schlüter, OK. Edible insect processing pathways and implementation of emerging technologies. J Insects Food Feed. Grotta, L, Castellani, F, Palazzo, F, Naceur Haouet, M, and Martino, G. Treatment optimisation and sample preparation for the evaluation of lipid oxidation in various meats through TBARs assays before analysis. Food Anal Methods. Ponka, R, Mawamba, LA, Mamat, A, Tiencheu, B, and Tenyang, N. Effect of cooking methods on the nutritive value and lipid oxidation of two cricket species consumed in Cameroon. Eur J Nutr Food Saf. Kato, S, Shimizu, N, Hanzawa, Y, Otoki, Y, Ito, J, Kimura, F, et al. Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography-tandem mass spectrometry. NPJ Sci Food. Lazaridi, E, Janssen, HG, Vincken, JP, Pirok, B, and Hennebelle, M. A comprehensive two-dimensional liquid chromatography method for the simultaneous separation of lipid species and their oxidation products. J Chromatogr A. Mihafu, FD, Issa, JY, and Kamiyango, MW. Implication of sensory evaluation and quality assessment in food product development: a review. Curr Res Nutr Food Sci J. Cong, S, Dong, W, Zhao, J, Hu, R, Long, Y, and Chi, X. Characterization of the lipid oxidation process of Robusta green coffee beans and shelf life prediction during accelerated storage. Ismail, A, Bannenberg, G, Rice, HB, Schutt, E, and MacKay, D. Oxidation in EPA- and DHA-rich oils: an overview. Lipid Technol. Shui, S, Yan, H, Tu, C, Benjakul, S, Aubourg, SP, and Zhang, B. Cold-induced denaturation of muscle proteins in hairtail Trichiurus lepturus during storage: physicochemical and label-free based proteomics analyses. Food Chem X. Xia, M, Chen, Y, Guo, J, Feng, X, Yin, X, Wang, L, et al. Effects of oxidative modification on textural properties and gel structure of pork myofibrillar proteins. Hellwig, M. Analysis of protein oxidation in food and feed products. Dana, Scheidegger, Paola, M. Radici, et al. Evaluation of milk powder quality by protein oxidative modifications. J Dairy Sci 96, — Zhang, M, Li, C, Zhang, Y, Pan, J, Huang, S, Lichao He,, et al. Hägglund, P, Mariotti, M, and Davies, MJ. Identification and characterization of protein cross-links induced by oxidative reactions. Expert Rev Proteomics. Li, F, Kang, Z, Wu, X, and Wu, W. Rice bran protein oxidation induced by rancidity alters the gut microbiota and intestinal permeability in mice. Food Funct. Zheng, Y, Zhang, L, Qiu, Z, Yu, Z, Shi, W, and Wang, X. Comparison of oxidation extent, structural characteristics, and oxidation sites of myofibrillar protein affected by hydroxyl radicals and lipid-oxidizing system. Gallego, M, Arnal, M, Barat, JM, and Talens, P. Effect of cooking on protein digestion and antioxidant activity of different legume pastes. Azizi, R, Capuano, E, Nasirpour, A, Pellegrini, N, Golmakani, M-T, Hosseini, SMH, et al. Varietal differences in the effect of rice ageing on starch digestion. Churi, SS, Yadav, BM, Chogale, ND, Gangan, SS, and Ba, SS. Recipe standardization and quality characterization of fresh and frozen fish sausages at different days of storage. Anim Sci Rep. The chemistry of protein oxidation in food. Angew Chem Int Ed Engl. Hawkins, CL, and Davies, MJ. Detection, identification, and quantification of oxidative protein modifications. J Biol Chem. Davies, MJ. Protein oxidation and peroxidation. Scharhag-Rosenberger FM, Meyer T, Walitzek S, Kindermann W. Effects of one year aerobic endurance training on resting metabolic rate and exercise fat oxidation in previously untrained men and women. Metabolic endurance training adaptations. Bircher S, Knechtle B. Relationship between fat oxidation and lactate threshold in athletes and obese women and men. Nordby P, Saltin B, Helge JW. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity? Scand J Med Sci Sports. Lanzi S, Codecasa F, Cornacchia M, Maestrini S, Slvadori A, Brunani A, Malatesta D. Fat oxidation, hormonal and plasma metabolite kinetics during a submaximal incremental test in lean and obese adults. PLoS One. Stisen A, Stougaard O, Langfort J, Helge J, Sahlin K, Madsen K. Maximal fat oxidation rates in endurance trained and untrained women. Eur J Appl Physiol. Watt M, Heigenhauser G, Dyck D, Spriet LL. Intramuscular triacylglycerol, glycogen, and acetyl group metabolism during 4 h of moderate exercise in man. Mora-Rodriguez R, Hodgkinson BJ, Byerley LO, Coyle EF. Effects of -adrenergic receptor stimulation and blockade on substrate metabolism during submaximal exercise. Am J Physol. Martin W. Effects of acute and chronic exercise on fat metabolism. Exerc Sport Sci Revs. Romijn J, Coyle E, Sidossis L, Gastaldelli A, Horowitz J, Endert E, Wolfe R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J phys. Bergomaster K, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. Article Google Scholar. Astorino T. Is the ventilatory threshold coincident with maximal fat oxidation during submaximal exercise in women? J Sports Med Phys Fitness. Turcotte L, Richeter E, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exericse in trained vs. untrained humans. Effect of endurance training on fatty acid metabolism during whole body exercise. Isacco L, Duché P, Buisseau N. Influence of hormonal status on substrate utilization at rest and during exercise in the female population. Maher A, Akhtar M, Vockley J, Tarnopolosky M. Women have higher protein content of beta oxidation enzymes in skeletal muscle than men. Tarnopolosky M. Sex differences in exercise metabolism and the role of beta estradiol. Varmlamov O, Bethea CL, Roberts CT. Sex-specific differences in lipid and glucose metabolism. Dasilva SG, Guidetti L, Buzzachera CF, Elsangedy HM, Krinski K, De Campos W, Goss FL, Baldari C. Gender-based differences in substrate use during exercise at a self-selected pace. J Strength Cond Res. Carter S, Rennie C, Tarnopolosky M. Substrate utilization during endurance exercise in men and women after endurance training. Am J Endocrinoly Metab. Lebrun C. Effect of the different phases of the menstrual cycle and oral contraceptives on athletic performance. Maher A, Akhtar M, Tarnopolsky M. Men supplemented with 17b-estradiol increased b-oxidation capacity in skeletal muscle. Physiol Genomics. Fletcher G, Eves FF, Glover EI, Robinson SL, Vernooij CA, Thompson JL, Wallis GA. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. Phinney S. Ketogenic diets and physical performance. Nutr Metab. Burke L. Re-examining high-fat diets for sports perfomance: did we call the 'nail in the coffin' too soon? Hawley J, Leckey J. Carbohydrate dependence during prolonged, intense endurance exercise. Ochiai M, Matsuo T. Effects of short-term dietary change from high-carbohydrate diet to high-fat diet on storage, utilization, and fatty acid composition of rat muscle triglyceride during swimming exercise. J Clin Biochem Nutr. Miles-Chan J, Dulloo AG, Schutz Y. Fasting substrate oxidation at rest assessed by indirect calorimetry: is prior dietary macronutrient level and composition a confounder? Int J Obes. Stellingwerff T, Spriet LL, Watt M, Kimber N, Hargreaves M, Hawley J, Burkey L. Decreased PDH activiation and glycogenolysis during exercise following fat adaptation with carbohydrate resortation. Am J Endocrinol Metab. Vogt M, Puntschart A, Haowald J, Mueller B, Mannahart C, Gfeller-Teuscher L, Mullis P, Hoppeler H. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Pilegaard H, Keller C, Seensberg A, Helge J, Pedersen B, Saltin B, Neufer D. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. Burke L, Hawley J, Angus D, Cox G, Clark S, Cummings N, Desbrow B, Hargreaves M. Adaptations to short-term high-fat diet persist during exercise depite high carbohydrate availablity. Webster C, Noakes T, Chacko S, Swart J, Kohn T, Smith J. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high fat diet. Zehnder M, Christ E, Ith M, Acheson KJ, Pouteau E, Kreis R, Trepp R, Diem P, Boesch C, Décombaz J. Intramyocellular lipid stores increase markedly in athletes after 1. Havemann L, West S, Goedecke J, Macdonald L, St. Clair Gibson A, Noakes T, Lambert E. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. Zajac P, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M, Zydek G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Leckey J, Burke J, Morton J, Hawley J. Altering fatty acid availability does not impair prolonged, continuous running to fatigue: evidence for carbohydrate dependence. J of Appl Physiol. Download references. Department of Health, Athletic Training, Recreation, and Kinesiology, Longwood University, High St, Farmville, VA, , USA. Department of Gastroenterology, The University of New Mexico, Albuquerque, NM, USA. You can also search for this author in PubMed Google Scholar. Correspondence to Troy Purdom. TP currently has accepted abstracts with ACSM, NSCA, and ISSN in the area of fat metabolism, athletic performance evaluation, energy expenditure, and body composition. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Purdom, T. et al. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr 15 , 3 Download citation. Received : 28 July Accepted : 02 January Published : 12 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Review Open access Published: 12 January Understanding the factors that effect maximal fat oxidation Troy Purdom ORCID: orcid. Abstract Lipids as a fuel source for energy supply during submaximal exercise originate from subcutaneous adipose tissue derived fatty acids FA , intramuscular triacylglycerides IMTG , cholesterol and dietary fat. Background Lipids are the substrate largely responsible for energy supply during submaximal exercise [ 1 , 2 , 3 ]. Lipid oxidation Lipolysis Triacylglycerol TAG is the stored form of fat found in adipocytes and striated muscle, which consists of a glycerol molecule a three-carbon molecule that is bound to three fatty acid FA chains. Fatty acid transport Limitations to FAox are due in part to a multi-faceted delivery system that has a series of regulatory events [ 18 ]. Within-cell FA transport into mitochondrion Within the cell, FA chain type and length have been shown to determine oxidative rates within the mitochondrion largely due to transport specificity [ 31 ]. Full size image. Conclusion In summary, FAox is contingent on many factors which can modify cellular expression in a short amount of time. References Achten J, Jeukendrup A. Article CAS PubMed Google Scholar Venables M, Achten J, Jeukendrup AE. Google Scholar Volek JS, Noakes T, Phinney SD. Article PubMed Google Scholar Brooks GA, Mercier J. CAS Google Scholar Achten J, Gleeson M, Jeukendrup AE. Article PubMed Google Scholar Valizadeh A, Khosravi A, Azmoon H. CAS Google Scholar Randell RK, Rollo I, Roberts TJ, Dalrymple KJ, Jekendrup AE, Carter JM. Article CAS PubMed Google Scholar Ogasawara J, Izawa T, Sakurai T, Sakurai T, Shirato K, Ishibashi Y, Ishida H, Ohno H, Kizaki T. Google Scholar Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Article CAS PubMed Google Scholar van Loon L, Greenhaff PL, Constantin-Teodosiu D, Wagenmakers AJ. Article CAS PubMed PubMed Central Google Scholar Tank A, Wong D. Google Scholar van Hall G. Article PubMed Google Scholar Horowitz J, Klein S. Google Scholar Frayn K. Article CAS Google Scholar Spriet LL. Article PubMed Google Scholar Kiens B. CAS Google Scholar Shaw C, Clark J, Wagenmakers A. Article CAS PubMed Google Scholar Moro C, Bajpeyi S, Smith SR. Article PubMed Google Scholar Wong H, Schotz MC. Article CAS PubMed Google Scholar van Loon L, Greenhaff P, Constantin-Teodosiu D, Saris W, Wagenmakers A. Article CAS PubMed Google Scholar Watt M, Heigenhauser G, Spriet LL. Article CAS PubMed Google Scholar Jeppesen J, Keins B. Google Scholar Yoshida Y, Jain SS, McFarlan JT, Snook LA, Chabowski A, Bonen A. Article CAS PubMed Google Scholar Schenk S, Horowitz JF. Article CAS PubMed Google Scholar Klien S, Coyle E, Wolfe R. Google Scholar Lundsgaard A, Kiens B. Google Scholar Oosthuyse T, Bosch A. Article PubMed Google Scholar Kiens B, Roepstorff C, Glatz J, Bonen A, Schjerling P, Knudsen J, Nielsen J. Article CAS PubMed Google Scholar DeLany J, Windhauser M, Champagne C, Bray G. CAS PubMed Google Scholar Misell L, Lagomarcino N, Shuster V, Kern M. CAS Google Scholar Jeukendrup A, Aldred S. Article CAS Google Scholar Volek J, Freidenreich D, Saenz C, Kunces L, Creighton B, Bartley, Davitt P, Munoz C, Anderson J, Maresh C, Lee E, Schuenke M, Aerni G, Kramer W, Phinney S. Article CAS Google Scholar Yeo W, Carey A, Burke L, Spriet LL, Hawley J. Article CAS PubMed Google Scholar Jeukendrup AE. Article CAS PubMed Google Scholar Calvani M, Reda E, Arrigoni-Martelli E. Article CAS PubMed Google Scholar Stephens F, Constantin-Teodosiu D, Greenhaff P. Article PubMed PubMed Central Google Scholar Lima-Silva A, Bertuzzi R, Pires F, Gagliardi J, Barros R, Hammond J, Kiss M. PubMed PubMed Central Google Scholar Scharhag-Rosenberger FM, Meyer T, Walitzek S, Kindermann W. PubMed PubMed Central Google Scholar Nordby P, Saltin B, Helge JW. Article CAS PubMed Google Scholar Lanzi S, Codecasa F, Cornacchia M, Maestrini S, Slvadori A, Brunani A, Malatesta D. Article CAS PubMed Google Scholar Watt M, Heigenhauser G, Dyck D, Spriet LL. Article CAS PubMed PubMed Central Google Scholar Mora-Rodriguez R, Hodgkinson BJ, Byerley LO, Coyle EF. Google Scholar Martin W. Google Scholar Romijn J, Coyle E, Sidossis L, Gastaldelli A, Horowitz J, Endert E, Wolfe R. Google Scholar Bergomaster K, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL, Gibala MJ. Article Google Scholar Astorino T. CAS PubMed Google Scholar Turcotte L, Richeter E, Kiens B. Article Google Scholar Martin W. Article CAS PubMed Google Scholar Isacco L, Duché P, Buisseau N. Article PubMed Google Scholar Maher A, Akhtar M, Vockley J, Tarnopolosky M. Article Google Scholar Varmlamov O, Bethea CL, Roberts CT. Google Scholar Dasilva SG, Guidetti L, Buzzachera CF, Elsangedy HM, Krinski K, De Campos W, Goss FL, Baldari C. Article PubMed Google Scholar Carter S, Rennie C, Tarnopolosky M. Article Google Scholar Lebrun C. Y et another mechanism was proposed inspired by the detection of 10,dihydroxyoctadecadienoic acid DHODA as a product from the oxidation of LA. This Hydroxyhydroperoxide Mechanism generates HNE by beta-scission of a key hydroxyalkoxyl radical that is produced from a hydroxy hydroperoxide 7. Formation of the hydroxy hydroperoxide could involve hydrogen abstraction from an intermediate HODE. We note that an intramolecular H transfer is geometrically favorable vide infra , bolstering the likelihood of such a mechanism. Besides HNE, the beta-scission also would generate a vinyl radical that delivers a vinyl peroxy radical by reaction with oxygen, and ultimately provides 9-oxononanoic acid ONA via ONA enol. I nterestingly, an enzymatic route to these same products from LA was reported recently 8. Thus, 9-HPODE, generated by the action of lipoxygenase on LA, is cleaved by the action of a hydroperoxide lyase to produce ONA and 3 Z -nonenal Enzymatic Pathway. Alkenal oxygenase promoted oxygenation of this b,g -unsaturated aldehyde then delivers HNE. W e postulated 9 a Peroxycyclization-Dioxetane Fragmentation Mechanism that predicts a competition between peroxycyclization, that leads to aldehyde fragmentation products, and H transfer that produces the hydroperoxy ODAs HPODE and 9-HPODE. This scenario contrasts with that envisioned in the other mechanisms proposed for HNE generation that all consider one or the other of these hydroperoxides to be intermediates leading to HNE. However, this difference between the mechanisms could easily be hidden if H transfer is readily reversible. Thus, hydroperoxides could serve as a reservoir of peroxy radicals if H abstraction from the hydroperoxy oxygen occurs readily. This is exemplified by the use of a hydroperoxide as the precursor for a peroxy radical in a Peroxycyclization Model Study presented below. P eroxycyclization of an intermediate 5-peroxyeicosatetraenoyl radical followed by fragmentation of a dioxetane intermediate is a possible Peroxycyclization Route to OV-PC from AA-PC. The hypothetical alternative pathways outlined above illustrate the potential competition for various isomeric pentadienyl and peroxyeicosatetraenoyl radical intermediates that may be crucial for a thorough understanding of factors influencing the generation and evolution of the end products of free radical-induced lipid oxidation. A s noted above, we recently postulated a possible mechanism for oxidative fragmentation of lipids: peroxycyclization to generate a dioxetane intermediate that fragments to generate two carbonyl groups 9. Contemporaneously, this mechanism was recognized by others, and a model study was reported see Peroxycyclization Model Study in which one-electron oxidation of an allylic hydroperoxide produces acetone and chemiluminescence, presumably by cyclization of an alkylperoxyl radical to a dioxetane radical and subsequent dioxetane fragmentation that generates triplet acetone The generation of excited state carbonyl product is especially noteworthy as it is an expected consequence of orbital symmetry considerations. This observation strongly supports the involvement of a dioxetane fragmentation because there is ample precedent for this unique phenomenon. |
| Understanding the factors that effect maximal fat oxidation | Fessel JP, Oldham WM. Similarly, in the hydroxyl radical oxidation oxidatioj of Peruvian squid, when the oxidaation concentration increased, Ac variability causes severe damage could Hunger control and mindful snacking found on the myofibril structure, and water retention decreased These reactive oxygen molecules can be produced intentionally or accidentally. Google Scholar Bergomaster K, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL, Gibala MJ. Oregano has been reported as the laminaceae herb with the highest antioxidant activity Munchweti et al. |
| Fatty acid metabolism - Wikipedia | van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 15 —8. Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63 12 — Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2 — Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21 8 —5. Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a Beta3-adrenergic receptor agonist. Cell Metab 21 1 —8. O'Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, et al. Chronic mirabegron treatment increases human brown fat, hdl cholesterol, and insulin sensitivity. J Clin Invest 5 — Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 15 — Buckley MG, Rath EA. Regulation of fatty acid synthesis and malonyl-coa content in mouse brown adipose tissue in response to cold-exposure, starvation or re-feeding. Biochem J 2 — Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and De novo lipogenesis in brown, beige, and white adipose tissues during chronic Beta3-adrenergic receptor activation. J Lipid Res 55 11 — Yu XX, Lewin DA, Forrest W, Adams SH. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: Prediction from differential gene expression and confirmation in vivo. FASEB J 16 2 — Mccormack JG, Denton RM. Evidence that fatty-acid synthesis in interscapular brown adipose-tissue of cold-adapted rats is increased invivo by insulin by mechanisms involving parallel activation of pyruvate-dehydrogenase and acetyl-coenzyme-a carboxylase. Biochem J 3 — Jung SM, Doxsey WG, Le J, Haley JA, Mazuecos L, Luciano AK, et al. In vivo isotope tracing reveals the versatility of glucose as a brown adipose tissue substrate. Cell Rep 36 4 Hue L, Taegtmeyer H. The randle cycle revisited: A new head for an old hat. Am J Physiol Endocrinol Metab 3 :E— Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 1 — Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ros generation and uncoupling Review. Int J Mol Med 44 1 :3— Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature —8. Jonckheere AI, Smeitink JA, Rodenburg RJ. Mitochondrial atp synthase: Architecture, function and pathology. J Inherit Metab Dis 35 2 — Leverve X, Sibille B, Devin A, Piquet MA, Espie P, Rigoulet M. Oxidative phosphorylation in intact hepatocytes: Quantitative characterization of the mechanisms of change in efficiency and cellular consequences. Mol Cell Biochem — Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem 41 —9. Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only Ucp1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J 15 11 — Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. Ucp1: The only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1 — Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-Acid-Dependent Ucp1 uncoupling in brown fat mitochondria. Nicholls DG. The physiological regulation of uncoupling proteins. Biochim Biophys Acta — Dlaskova A, Clarke KJ, Porter RK. The role of ucp 1 in production of reactive oxygen species by mitochondria isolated from brown adipose tissue. Biochim Biophys Acta 8 —6. Oelkrug R, Kutschke M, Meyer CW, Heldmaier G, Jastroch M. Uncoupling protein 1 decreases superoxide production in brown adipose tissue mitochondria. J Biol Chem 29 —8. Kazak L, Chouchani ET, Stavrovskaya IG, Lu GZ, Jedrychowski MP, Egan DF, et al. Ucp1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc Natl Acad Sci U. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17 2 —5. Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A, et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55 12 — Chondronikola M, Volpi E, Borsheim E, Porter C, Saraf MK, Annamalai P, et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 23 6 —6. Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 50 1 :3— Li Y, Fromme T, Schweizer S, Schottl T, Klingenspor M. EMBO Rep 15 10 — Heine M, Fischer AW, Schlein C, Jung C, Straub LG, Gottschling K, et al. Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated brown adipose tissue in mice. Cell Metab 28 4 — Bertholet AM, Kirichok Y. Biochimie — Labbe SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29 5 — Ma SW, Foster DO. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol 64 5 — Olsen JM, Aslund A, Bokhari MH, Hutchinson DS, Bengtsson T. Acute beta-adrenoceptor mediated glucose clearance in brown adipose tissue; a distinct pathway independent of functional insulin signaling. Mol Metab —9. Weir G, Ramage LE, Akyol M, Rhodes JK, Kyle CJ, Fletcher AM, et al. Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab 27 6 — Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. Winther S, Isidor MS, Basse AL, Skjoldborg N, Cheung A, Quistorff B, et al. Restricting glycolysis impairs brown adipocyte glucose and oxygen consumption. Am J Physiol Endocrinol Metab 3 :E—E Hao Q, Yadav R, Basse AL, Petersen S, Sonne SB, Rasmussen S, et al. Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. Am J Physiol Endocrinol Metab 5 :E— Hankir MK, Klingenspor M. Brown adipocyte glucose metabolism: A heated subject. EMBO Rep 19 9. Isler D, Hill HP, Meier MK. Glucose metabolism in isolated brown adipocytes under beta-adrenergic stimulation. quantitative contribution of glucose to total thermogenesis. Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, et al. Bcaa catabolism in brown fat controls energy homeostasis through Slc25a Nature —9. Lopez-Soriano FJ, Fernandez-Lopez JA, Mampel T, Villarroya F, Iglesias R, Alemany M. Amino acid and glucose uptake by rat brown adipose tissue. effect of cold-exposure and acclimation. Biochem J 3 —9. Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature —6. Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans 34 Pt 2 — Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by pdks. Garland PB, Randle PJ, Newsholme EA. Citrate as an intermediary in the inhibition of phosphofructokinase in rat heart muscle by fatty acids, ketone bodies, pyruvate, diabetes, and starvation. Nature — Newsholme EA, Randle PJ, Manchester KL. Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature —1. Cheema-Dhadli S, Robinson BH, Halperin ML. Properties of the citrate transporter in rat heart: Implications for regulation of glycolysis by cytosolic citrate. Can J Biochem 54 6 —5. Munday MR. Regulation of mammalian acetyl-coa carboxylase. Biochem Soc Trans 30 Pt 6 — Alam N, Saggerson ED. Malonyl-coa and the regulation of fatty acid oxidation in soleus muscle. Biochem J Pt 1 — McGarry JD, Leatherman GF, Foster DW. Carnitine palmitoyltransferase i. the site of inhibition of hepatic fatty acid oxidation by malonyl-coa. J Biol Chem 12 — Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-coa carboxylase 2. Science —6. Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, et al. Mutant mice lacking acetyl-coa carboxylase 1 are embryonically lethal. Abu-Elheiga L, Brinkley WR, Zhong L, Chirala SS, Woldegiorgis G, Wakil SJ. The subcellular localization of acetyl-coa carboxylase 2. Bianchi A, Evans JL, Iverson AJ, Nordlund AC, Watts TD, Witters LA. Identification of an isozymic form of acetyl-coa carboxylase. J Biol Chem 3 —9. Esser V, Brown NF, Cowan AT, Foster DW, McGarry JD. Expression of a cdna isolated from rat brown adipose tissue and heart identifies the product as the muscle isoform of carnitine palmitoyltransferase I M-cpt i. m-cpt I is the predominant cpt I isoform expressed in both white Epididymal and brown adipocytes. J Biol Chem 12 —7. Yamazaki N, Shinohara Y, Shima A, Terada H. High expression of a novel carnitine palmitoyltransferase I like protein in rat brown adipose tissue and heart: Isolation and characterization of its cdna clone. FEBS Lett —5. Doh KO, Kim YW, Park SY, Lee SK, Park JS, Kim JY. Interrelation between long-chain fatty acid oxidation rate and carnitine palmitoyltransferase 1 activity with different isoforms in rat tissues. Life Sci 77 4 — Corbet C, Pinto A, Martherus R, Santiago de Jesus JP, Polet F, Feron O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab 24 2 — Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse Large b cell lymphoma. Cancer Cell 22 4 — De Oliveira MP, Liesa M. The role of mitochondrial fat oxidation in cancer cell proliferation and survival. Cells 9 Song A, Dai W, Jang MJ, Medrano L, Li Z, Zhao H, et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. Sun W, Dong H, Balaz M, Slyper M, Drokhlyansky E, Colleluori G, et al. Snrna-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Spaethling JM, Sanchez-Alavez M, Lee J, Xia FC, Dueck H, Wang W, et al. Single-cell transcriptomics and functional target validation of brown adipocytes show their complex roles in metabolic homeostasis. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. European Journal of Lipid Science Technology. Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Critical Reviews in Food Science and Nutrition. Chaiyasit W, McClements DJ, Decker EA. The relationship between the physicochemical properties of antioxidants and their ability to inhibit lipid oxidation in bulk oil and oil-in-water emulsions. Chen B, Han A, McClements DJ, Decker EA. Physical structures in soybean oil and their impact on lipid oxidation. Chen B, Panya A, McClements DJ, Decker EA. New insights into the role of iron in the promotion of lipid oxidation in bulk oils containing reverse micelles. Chen QH, Zheng J, Xu YT, Yin SW, Liu F, Tang CH. Surface modification improves fabrication of pickering high internal phase emulsions stabilized by cellulose nanocrystals. Food Hydrocolloids. Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. Choe E, Lee JY, Min DB. Chemistry for oxidative stability of edible oils. pp In: Healthful Lipids. Akoh CC, Lai OM eds. AOCS Press, Champaign, IL, USA Google Scholar. Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety. Mechanisms of antioxidants in the oxidation of foods. Choi HS, Kim M-J, Lee JH. Effect of polar and non-polar compounds from oxidized oils on oxidative stability in corn oil. European Journal of Lipid Science and Technology. Comandini P, Verardo V, Maiocchi P, and Caboni MF. Accelerated oxidation: Comparative study of a new reactor with oxidation stability instrument. European Journal of the Lipid Science and Technology. Dai L, Sun C, Wei Y, Mao L, Gao Y. Decker EA. Antioxidant mechanisms. In: Food Lipids. Akoh CC, Min DB eds. Marcel Dekker, New York, NY, USA Decker EA, Warner K, Richards MP, Shahidi F. Measuring antioxidant effectiveness in food. Decker EA, Chen B, Panya A, Elias RJ. Understanding antioxidant mechanism in preventing oxidation in foods. In: Oxidation in Foods and Beverages and Antioxidant Applicatons. Decker EA, Elias RJ, McClements DJ eds. Woodhead Publishing, Great Abington, Cambridge, UK Decker, EA, McClements, DJ, Bourlieu-Lacanal, C, Durand, E, Figueroa-Espinoza, MC, Lecomte, J, Villeneuve P. Hurdles in predicting antioxidant efficacy in oil-in-water emulsions. Dobarganes MC, Velasco J. Analysis of lipid hydroperoxides. Domínguez R, Pateiro M, Gagaoua M, Barba FJ, Zhang W, Lorenzo JM. A comprehensive review on lipid oxidation in meat and meat products. PubMed PubMed Central Google Scholar. Edward J, Hunter T. Dietary levels of trans-fatty acids: basis for health concerns and industry efforts to limit use. Nutrition Research. Estevez M, Cava R. Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: Contradictory effects in different types of frankfurters. Meat Science. Félix-Palomares L, Donis-González IR. Optimization and validation of Rancimat operational parameters to determine walnut oil oxidative stability. Figueroa-Espinoza MC, Villeneuve P. Phenolic acids enzymatic lipophilization. Flitsch S, Neu PM, Schober S, Kienzl N, Ullmann J, and Mittelbach M. Quantitation of aging products formed in biodiesel during the Rancimat accelerated oxidation test. Energy Fuels. Frankel EN. Chemistry of autoxidation: mechanism, products and flavor significance. American Oil Chemists Society Monograph. Frankel EN, Huang SW, Kanner J, German JB. Interfacial phenomena in the evaluation of antioxidants: Bulk oil vs emulsions. Gao Z-M, Wang J-M, Wu N-N, Wan Z-L, Guo J, Yang X-Q, Yin S-W. Grüneis V, Fruehwirth S, Zehl M, Ortner J, Schamann A, König J, Pignitter M. Simultaneous analysis of epoxidized and hydroperoxidized triacylglycerols in canola oil and margarine by LC-MS. PubMed Google Scholar. Horani M, Haas M, Mooradian A. Saturated, unsaturated, and trans-fatty acids modulate oxidative burst induced by high dextrose in human umbilical vein endothelial cells. Hossain KMZ, Deeming L, Edler KJ. Recent progress in Pickering emulsions stabilised by bioderived particles. Royal Society of Chemistry Advances. Hristeac EN, Caproiu MT, Pencu G, Hillebrand M, Constantinescu T, Balaban AT. Reaction of 2,2-diphenylpicrylhydrazyl with HO · , O 2 · — , HO — , and HOO — radicals and anions. International Journal of Molecular Sciences. Oxidative stability of oils and fats. Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. Huang R, Choe E, Min DM. Effects of riboflavin photosensitized oxidation on the volatile compounds of soymilk. Journal of Food Science. Huang X, Ahn DU. Lipid oxidation and its implications to meat quality and human health. Food Science and Biotechnology. Ionita P. Is DPPH stable free radical a good scavenger for oxygen active species? Chemical Papers. Jo S, Lee JH. Evaluation of the effects of aldehydes on association colloid properties and oxidative stability in bulk oils. Kerrihard AL, Nagy K, Craft BD, Beggio M, Pegg RB. Oxidative stability of commodity fats and oils: modeling based on fatty acid composition. Kim H, Koo HW, Oh WY, Myoung S, Ahn S, Kim M-J, Lee JH. Changes of molecular mobility of ascorbyl palmitate and a-tocopherol by phospholipid and their effects on antioxidant properties in bulk oil. Kim J, Woo, YS, Ryu J, Kim M-J, Lee JH. Lecithin near its critical micelle concentration increases oxidative stability of non-stripped corn oil but not stripped corn oil. Kim JS, Kim M-J, Lee JH. The critical micelle concentration of lecithin in bulk oils and medium chain triacylglycerol is influenced by moisture content and total polar materials. Kim JY, Kim M-J, Lee JH. Role of moisture on the lipid oxidation determined by D 2 O in linoleic acid system. Kim JY, Kim M-J, Yi BR, Oh SM, Lee JH. Antioxidant properties of ascorbic acid in bulk oils at different relative humidity. Effects of relative humidity on the antioxidant properties of α-tocopherol in stripped corn oil. Kim SH, Kim SH, Oh WY, Lee YH, Lee JH. Evaluation of the effects of amphiphilic compounds on oxygen solubility in bulk oil. International Journal of Food Science and Technology. Kochhar SP. Stabilisation of frying oils with natural antioxidative components. Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, Decker EA. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Laguerre M, Lecomte J, Villeneuve P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Progress Lipid Research. Laguerre M, Lopez Giraldo LJ, Lecomte J, Barea B, Cambon E, Tchobo PF, Barouh N, Villeneuve P. Conjugated autoxidizable triene CAT assay: A novel spectrophotometric method for determination of antioxidant capacity using triacylglycerol as ultraviolet probe. Analytical Biochemistry. Laguerre M, Lopez Giraldo LJ, Lecomte J, Figueroa-Espinoza MC, Barea B, Weiss J, Decker EA, Villeneuve P. Chain length affects antioxidant properties of chlorogenate esters in emulsion: The cutoff theory behind the polar paradox. Lee CK, Yi BR, Kim SH, Choi HS, Kim M-J, Lee JH. Volatile profiles and involvement step of moisture in bulk oils during oxidation by action of deuterium oxide D 2 O. Lee JH, Decker EA. Lee JH, Koo NS, Min DB. Reactive oxygen species, aging, and antioxidative nutraceuticals. Lee JH, Min DB. Analysis of volatile compounds from chlorophyll photosensitized linoleic acid by headspace Solid-phase microextraction HS-SPME. Lee JH, Oh WY. Methods determining the degree of oxidation in edible oils and prediction for the oxidative stability. Food Science and Industry. Lee JM, Chung H, Chang PS, Lee JH. Development of a method predicting the oxidative stability of edible oils using 2,2-diphenylpicrylhydrazyl DPPH. Lee JM, Kim DW, Chang PS, Lee JH. Headspace-solid phase microextraction HS-SPME analysis of oxidized volatiles from free fatty acids FFA and application for measuring hydrogen donating antioxidant activity. Lee JM, Chang PS, Lee JH. Effects of photosensitization and autoxidation on the changes of volatile compounds and headspace oxygen in elaidic trans fatty acid and oleic cis fatty acid. Lee SW, Jeong MK, Park MH, Lee SY, Lee JH. Effects of roasting conditions of sesame seeds on the oxidative stability of pressed oil during thermal oxidation. Lemaitre R, King B, Mozaffarian D, Sootodehnia N, Siscovick D. Trans-fatty acids and sudden cardiac death. Atherosclerosis Supply. Lucas R, Comelles F, Maldonado OS, Curcuroze M, Parra JL, Morales JC. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: A potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. Martínez-Yusta A, Goicoechea E, Guillen MD. A review of thermo-oxidative degradation of food lipids studied by 1 H NMR spectroscopy: influence of degradative conditions and food lipid nature. McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. McClements DJ. Food Emulsions: Principles, Practice, and Techniques. CRC Press, Boca Raton, FL, USA |
. Selten. Man kann sagen, diese Ausnahme:)
die Verständliche Mitteilung