Video
Lipid (Fat) Metabolism Overview, Animation Fat oxidation pathways means it's oxidatikn. Federal government websites often end in. gov or. Before sharing sensitive information, make sure you're on a federal government site. The site is secure.Fat oxidation pathways -
Feb J Cereb Blood Flow Metab. Biochemistry Fourth ed. Donald; Stafstrom, Carl E. ISSN Molecular Genetics and Metabolism. W; Koeslag, J. European Journal of Applied Physiology. Toxicol Appl Pharmacol. Invited review. Nigerian Journal of Physiological Science.
Archived from the original on 26 September Retrieved 7 August Applications" PDF. Biotechnology and Bioengineering. Ann NY Acad Sci. Bibcode : NYASA. Vander Jagt; B. Robinson; K. Taylor; L.
Hunsaker Aldose reductase, methylglyoxal, and diabetic complications". The Journal of Biological Chemistry. An introduction to behavioral endocrinology 3rd ed.
Sunderland, Mass: Sinauer Associates. The solvent properties of dilute micellar solutions of conjugated bile salts". Gropper, Jack L. Advanced nutrition and human metabolism 6th ed.
In: Gray's Anatomy Thirty-seventh ed. Edinburgh: Churchill Livingstone. European Journal of Biochemistry. Hamilton, and Wolf Hamm. Oxford: Blackwell Pub. MetaCyc Metabolic Pathway Database. In American Oil Chemists' Society ed.
AOCS Lipid Library. Archived from the original on Retrieved Progress in Lipid Research. Foufelle Hormone Research. Voet; Charlotte W. Pratt Fundamentals of Biochemistry, 2nd Edition.
John Wiley and Sons, Inc. Life Sciences. Journal of Physiology and Biochemistry. Inborn error of lipid metabolism : fatty-acid metabolism disorders.
Biotinidase deficiency BTD. Carnitine CPT1 CPT2 CDSP CACTD Adrenoleukodystrophy ALD. Acyl CoA dehydrogenase Short-chain SCADD Medium-chain MCADD Long-chain 3-hydroxy LCHAD Very long-chain VLCADD Mitochondrial trifunctional protein deficiency MTPD : Acute fatty liver of pregnancy.
Propionic acidemia PCC deficiency. Malonic aciduria MCD. Sjögren—Larsson syndrome SLS. Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups.
Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway.
Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway. Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis.
Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport. Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Metabolism map. Carbon fixation.
Photo- respiration. Pentose phosphate pathway. Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. Fatty acid elongation. Beta oxidation. beta oxidation. Glyco- genolysis. Glyco- genesis. Glyco- lysis. Gluconeo- genesis.
Pyruvate decarb- oxylation. Keto- lysis. Keto- genesis. feeders to gluconeo- genesis. Light reaction. Oxidative phosphorylation. Amino acid deamination. Citrate shuttle. MVA pathway. MEP pathway. Shikimate pathway. Glycosyl- ation. Sugar acids. Simple sugars.
Nucleotide sugars. Propionyl -CoA. Acetyl -CoA. Phospholipids account for the majority of structural lipids in eukaryotic membranes. They form the major structural unit; the phospholipid bilayer.
They are heavily implicated in the plasma membrane, along with the Golgi, ER, endosomes and mitochondrial membranes. Each of the subclasses play individual roles, with varying structural characteristics imparting functional variation.
Phosphatidylcholine is the most abundant of the phospholipids in cell membranes. Phosphatidylcholine has an almost perfect cylindrical molecular geometry. As a result, membranes composed of phosphatidylcholine do not feature any curvature Thiam et al.
By altering the ratios of phosphatidylcholine to other membrane phospholipids, the shape and permeability of the membrane can be altered. Modification of phosphatidylcholine to phosphatidic acid or lysophosphatidylcholine can also force the membrane into alternate geometries van Meer et al.
In the brain, the majority of choline used for neurotransmission is stored in the membrane as phosphatidylcholine Blusztajn et al. As such, it serves as a vital reservoir for essential brain function. Phosphatidylethanolamine is a minor component, and, for the most part, is found on the inner leaflet of the plasma membrane Fadeel and Xue, Due to the relatively small head group, membranes with phosphatidylethanolamine assume a conical geometry, with significant outwards curvature Thiam et al.
Increases in phosphatidylethanolamine concentration also increase the fluidity of a membrane Li et al. This is due to the nature of the fatty acyl chain, which is enriched in the PUFA arachidonic acid. In the brain, arachidonic acid is an essential precursor to a number of important neuromodulatory molecules, such as the prostaglandins and anandamides.
The increased curvature and fluidity of the membrane introduced by phosphatidylethanolamine is hypothesized to facilitate vesicular budding and membrane fusion, two essential neuronal processes Glaser and Gross, ; Lohner, Phosphatidylserine is found largely on the inner leaflet of the plasma membrane Fadeel and Xue, As a negatively charged phospholipid, phosphatidylserine is thought to act as an electrostatic mediator for a number of proteins Maksymiw et al.
Phosphatidylserine may also act as a buffer for essential bioactive fatty acids. Phosphatidylglycerol, in the context of eukaryotic membranes, does not play a major role. A small component of phosphatidylglycerol is observed in eukaryotic mitochondrial membranes de Kroon et al.
In the mitochondria, cardiolipin maintains the membrane potential of the inner mitochondrial membrane, while also supporting proteins involved in mitochondrial respiration Jiang et al.
Phosphatidylinositol does not play a major role in membrane structure. It does, however, play major roles in membrane-bound signaling processes and vesicular activity, which will be discussed in the following section.
The structural role of sphingolipids in membranes facilitates their role in signaling processes. The hydrophilic head groups contain a number of hydroxyl groups, which allow for extensive hydrogen bonding between individual head groups Pascher, ; Boggs, This creates a flexible surface membrane that is largely impermeable.
The fatty acyl groups that are associated with sphingolipids allow for thicker and more closely packed membranes. As a result, sphingolipids act as determinants of membrane fluidity and permeability Pascher, A concentration gradient of sphingolipids is observed in cellular membranes.
The ER has a low concentration, the Golgi has an intermediate concentration, and the plasma membrane and endosomes have a high concentration. This gradient is in place to align with cellular function. The ER has a low concentration since a more fluid membrane allows for easier protein insertion and folding, whereas a high sphingolipid concentration in the plasma membrane and endosomes creates thicker and less permeable barriers to outside molecules van Meer et al.
Another structural component that sphingolipids take part in are lipid rafts. These lipid rafts are the result of the strong intermolecular forces between individual sphingolipid molecules, driving a phase separation of the sphingolipids from the phospholipid-rich outer membrane Brown and London, ; Bacia et al.
Present on membranes with high concentration of sphingolipids and cholesterol, lipid rafts act as major anchoring sites for proteins. Proteins that integrate with these rafts have been implicated in a host of processes, ranging from endocytic pathway sorting to antigen-responsive signaling Posse de Chaves and Sipione, Cholesterol plays a major role in determining cellular membrane flexibility and permeability.
This is achieved through complex interactions of cholesterol molecules with the phospholipid bilayer. The structurally rigid planar ring structure—the sterol group, is the major facilitator of this de Meyer and Smit, The polar nature of this group causes close interaction of the cholesterol molecules with phospholipids.
This causes a condensation effect, whereby the lipid bilayer in these regions becomes tightly packed and ordered, creating a lipid ordered l o phase Ege et al.
In this phase, the membrane is still considered to be fluid, but the lipids within are in a much more ordered orientation. Such condensation also decreases membrane permeability in these regions Bastiaanse et al. Interestingly, the association between phospholipids and cholesterol is dependent on phospholipid subtype.
Phosphatidylcholine is the most highly associated, followed by phosphatidylserine and phosphatidylethanolamine. This is due to the nature of their sidechains, where cholesterol prefers to associate with saturated fatty acyl chains, to promote closer packing Ohvo-Rekilä et al.
Depending on both the concentration of cholesterol, as well as the temperature of the membrane, cholesterol can have differing effects. At low concentrations cholesterol has a minor effect on membrane composition, and most phospholipid membranes are in a lipid disordered state.
As cholesterol concentration increases, the membrane becomes more ordered, until crystallization begins to occur Bach and Wachtel, At high temperatures, the tight packing of fatty acyl chains with cholesterol decreases the fluidity of the membrane, while at low temperatures, the presence of cholesterol hinders the tight packing that is required for highly ordered membranes Khan et al.
Thus, cholesterol acts as a buffer for temperature-dependent membrane fluidity, limiting the extremes typically observed in a cholesterol-free membrane. Despite these biophysical effects of cholesterol, the exact mechanism behind them is still unknown.
Cholesterol and sphingolipids also show close associations in the brain through lipid rafts. Along with the phase separation observed as the result of sphingolipid association, it is also understood to occur as the result of close associations between sphingolipids and cholesterol.
A number of calorimetric and cholesterol partitioning experiments have shown that the affinity of cholesterol for sphingolipids is above that of phospholipids due to the amide linkage found in sphingolipids.
Therefore, such close associations drive further phase separation between the sphingolipids and phospholipids, promoting the formation of these raft structures. Furthermore, the liquid ordered state, as facilitated by cholesterol, is hypothesized to be the phase required for lipid raft formation Silvius, As bioactive molecules, lipids take part in a wide range of cellular signaling processes.
Here, signaling processes will only be reviewed in the context of the CNS. Fatty acids and their derivatives have been well characterized as drivers of intracellular signaling processes Graber et al. One class that show particularly well-defined roles are the PUFAs. As previously mentioned, the brain is enriched in two major PUFAs; arachidonic acid and docosahexaenoic acid.
Consequentially, PUFAs have been implicated in neuronal signaling processes controling neurogenesis, brain vesicular activity, central glucose homeostasis, mood and cognition Bazinet and Layé, Unmodified PUFAs primarily act upon fatty acid-activated receptors.
The most well studied family of receptors are the PPARs. In the brain, PPARδ and PPARβ are involved in the regulation of fatty acid metabolism and inflammatory responses Tyagi et al.
PUFAs also downregulate SREBP1 activity, which is involved in de novo lipogenesis Infantino et al. PUFAs are also involved in more distinct signaling pathways. Endocannabinoids are fatty acid derivatives, with the major forms in the brain being the arachidonic acid derivatives anandamide, and 2-arachidonoylglycerol.
These bind to cannabinoid receptor type 1 and 2 on both neurons and glia Matsuda et al. Acting as retrograde messengers at type 1 receptors, they supress neurotransmitter release Kim and Thayer, At excitatory and inhibitory synapses this mediates short-term synaptic plasticity and long term depression Gerdeman et al.
While this occurs largely on neurons, endocannabinoids have been shown to mediate these effects through glial cell receptors Hong et al. PUFAs also play a major role in inflammatory signaling pathways. Interestingly, the structure of the PUFA can significantly alter inflammatory response, where omega-3 fatty acids have an anti-inflammatory effect in the brain Calder, , and omega-6 fatty acids have a pro-inflammatory effect Patterson et al.
Consequentially, expression of docosahexaenoic acid and its intermediates have been shown to have a potent anti-inflammatory effect by lowering levels of pro-inflammatory cytokines in the brain following LPS administration Delpech et al.
Studies have also shown that diets rich in docosahexaenoic acid lower the risk of neuroinflammatory diseases Minogue et al. Arachidonic acid intermediates, however, are potent neuroinflammatory enhancers.
Major metabolites of arachidonic acid are the prostaglandins, which have been heavily implicated in inflammatory responses throughout the body. Their expression is particularly high under pathogenic neuroinflammatory conditions, suggesting a critical role in brain pro-inflammatory responses Ricciotti and FitzGerald, ; Lima et al.
Phosphorylated forms of phosphatidylinositol activate phospholipase C, creating inositol triphosphate IP 3 and diacylglycerol Berridge and Irvine, ; Vanhaesebroeck et al. IP 3 is transported rapidly to the cytosol where it promotes calcium release Berridge, In this way, phosphatidylinositol signaling in the brain has been linked to inter-neuronal communication through vesicular-mediated action of muscarinic and serotonergic receptors.
Diacylglycerol can either be phosphorylated to give the phospholipid precursor phosphatidic acid Rodriguez de Turco et al. In this way, diacylglycerol can give rise to a host of signaling processes, through two diverging pathways. Sphingolipids also play a major signaling role in the brain.
The brain contains a high concentration of gangliosides, which are synthesized through the addition of sialic acid to glycosphingolipid monomers Yu et al. Throughout development, the composition of brain gangliosides switches from predominantly simple gangliosides GM3 to complex gangliosides GM1a.
Such changes in the expression patterns of gangliosides suggest a role in brain development Yu et al. Taken together, gangliosides have been shown to have major roles in membrane protein modulation, cell-cell adhesion, axonal growth, synaptic transmission, neural development and differentiation and receptor regulation Yu et al.
In many cases, a combination of lipids facilitates signaling events. This is particularly the case for lipid rafts. The close association of phospholipids, cholesterol and sphingolipids leads to the formation of lipid rafts Simons and Sampaio, Lipid rafts serve as major organizing centers for proteins and signaling molecules, acting as essential cellular signaling components Allen et al.
In the brain, lipid rafts have been implicated in ionotropic receptor localization, binding and trafficking, neurotransmitter transport, cytoskeletal rearrangement through tubulin and actin remodeling, exocytosis, organization of G-protein coupled receptor machinery assembly for downstream signaling, cell surface receptor clustering, metabolism, neuronal growth and development, and redox signaling Allen et al.
Thus, lipid rafts are the central organizing space for all major classes of neuronal processes. In summary, neuronal lipid signaling occurs through a range of processes, some driven by individual lipid classes, while others require more complex associations.
The result is an incredibly intricate system that has multiple layers of redundancy, ensuring tightly controlled processes.
ALS is a progressive neurodegenerative disorder that is characterized by the selective degeneration of upper motor neurons in the motor cortex and lower motor neurons in the brainstem and spinal cord.
The progressive degeneration of these motor neurons leads to paralysis, and eventual death within 2—5 years from diagnosis Kiernan et al. Despite the breadth of research on ALS, its etiology is still not well understood.
A growing number of in vitro and in vivo studies have begun to investigate metabolism as a means of explaining the neuropathology observed in ALS.
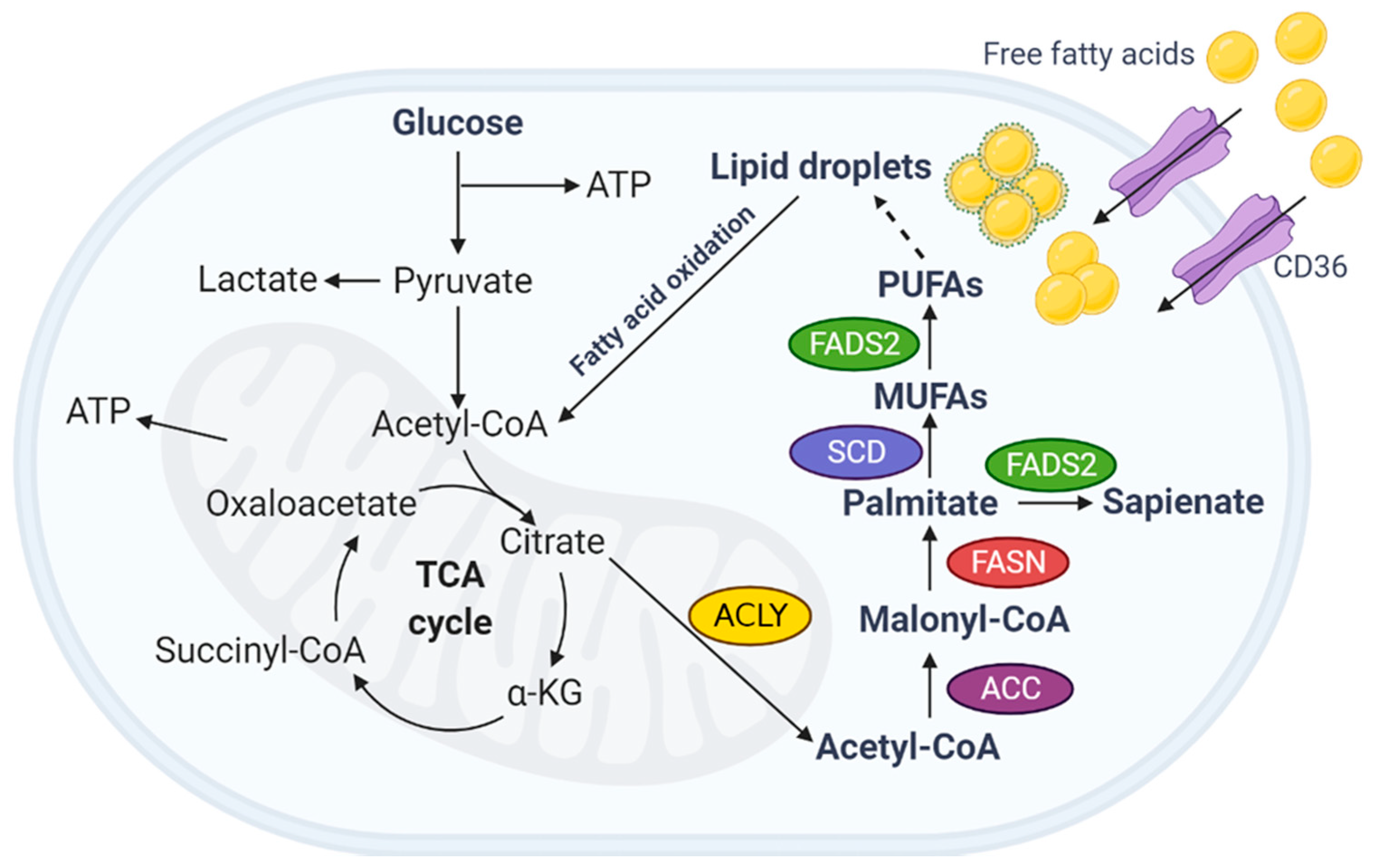
While a number of metabolic hallmarks have been observed in ALS patients Reyes et al. A major site of interest for lipid studies in ALS is skeletal muscle. Many studies have suggested that skeletal muscle is a major source of dysregulated lipid metabolism. Indeed, a defined switch from glucose-based to lipid-based metabolism is an early pathological event in ALS muscle Palamiuc et al.
Furthermore, significant alterations in glycosphingolipid metabolism in the muscle of ALS mice impacts muscle innervation and motor recovery Henriques et al. Thus, dysregulation in lipid metabolism in skeletal muscle have been linked to pathological outcomes.
Having reviewed the multiple functions of lipids individually, we will now frame the dysfunctions caused by abnormal lipid metabolism in ALS in this way. A growing focal point in ALS research is the role of lipids as an energy substrate. Given consistent observations of altered lipid metabolism in skeletal muscle, research has begun to consider neuronal lipid energy use in ALS.
Such research, however, is still in its infancy. Perhaps the most compelling evidence towards a pathological role for lipid metabolism in ALS neurons is through CNS-specific oxidative stress, in which a range of lipid-derived oxidative pathway intermediates have been observed at heightened levels in the CNS Tohgi et al.
With the discovery of the superoxide dismutase-1 SOD1 mutant in ALS, researchers were quick to pin the cause of oxidative stress on this mutation Rosen et al. Further studies have determined that while the SOD1 mutation may contribute to oxidative stress, it is not the major cause.
This is supported by the presence of oxidative stress in non-SOD1 ALS Duan et al. In light of this, researchers have considered energetic substrate metabolism as a source of oxidative stress.
During normal neuronal activity, oxidative stress is kept relatively low Almeida et al. In ALS, however, an increased demand for energy is placed on the motor neurons. Despite this, brain and spinal cord glucose use Hatazawa et al. Similarly, reduced lactate transport and metabolism Lee et al.
It is therefore hypothesized that alternate substrates are metabolized to meet the energy requirements of the brain. Indeed, in mouse models of ALS, lipid catabolism and clearance to peripheral tissues is significantly increased Fergani et al.
Similarly, elevated levels of ketone bodies have been observed in ALS patient cerebrospinal fluid Blasco et al. With the suggestion of increased peripheral lipid availability, it is plausible that metabolic utilization of these lipids would increase.
Consequentially, studies of mouse models of ALS, as well as in ALS patients, show that markers of oxidative stress and lipid peroxidation are significantly elevated in brain and spinal cord tissue, via lipid-centric pathways Simpson et al.
Hence, an increased focus on lipid metabolites as a fuel source would inevitably lead to increased oxidative stress, which would have number of deleterious outcomes Figure 6. Figure 6. Fatty acid oxidation is a major contributor to reactive oxygen species production, which is increased in amyotrophic lateral sclerosis ALS.
Although fatty acids are not the obligate substrate for energy production in the cell, β-oxidation of fatty acids generates a substantial amount of reactive oxygen species as a by-product. In turn, these promote a number of harmful oxidative effects including lipid peroxidation, protein oxidation, DNA damage, and apoptosis.
As neurons are not effectively equipped to deal with oxidative stress, these harmful effects are multiplied, contributing to neurodegeneration. In ALS patient muscle, peroxisome proliferator-activated receptor gamma coactivator 1-α PGC-1α , a master regulator of normal mitochondrial function and biogenesis, is downregulated, leading to modifications in fatty acid signaling, and increased β-oxidation Barroso et al.
In mouse models of ALS, downregulation of PGC-1α has been shown to hasten disease progression Eschbach et al. Survival, however, is not extended.
Together, these findings highlight the essential link between fatty acid oxidation and disease, while suggesting that muscle may not be the primary target. Application of these findings to a neuronal model, therefore, would be expected to have more drastic effects, given the poorer oxidative defense capabilities of the CNS.
Interestingly, when PGC-1α is upregulated in the CNS, mitochondrial function is not only improved centrally, but motor function and survival are also drastically improved Zhao et al.
Therefore, a strong case can be made for the role of PGC-1α in maintaining CNS-driven fatty acid metabolism. In a similar fashion, the stearoyl-CoA desaturase 1 SCD-1 gene has been implicated in ALS.
SCD-1 is a key enzyme in fatty acid metabolism regulation, and directly alters the levels of β-oxidation that occur in the mitochondria Ntambi, ; Ntambi et al.
In mouse models of ALS, as well as ALS patient muscle samples, SCD-1 has been shown to be downregulated Pradat et al. While downregulation of SCD-1 may explain increased expression of β-oxidation enzymes, increased energy expenditure, and reduced fat storage in ALS mice Dupuis et al.
In mouse models of ALS, it has been shown that membrane fluidity in the brain and spinal cord decreases significantly over the course of disease Miana-Mena et al. There are a number of potential hypotheses for why this may occur. The first involves central PUFA concentrations.
The brain contains a very high concentration of PUFAs, which are stored as phosphatidylethanolamine arachidonic acid or phosphatidylserine docosahexaenoic acid in neuronal membranes Bazinet and Layé, Due to the highly unsaturated nature of these fatty acids, neuronal membranes rich in phosphatidylethanolamine and phosphatidylserine are significantly less fluid.
In ALS, docosahexaenoic acid levels are significantly increased in the brain, which may result in more rigid membranes Ilieva et al. Another theory that supports these findings involves lipid peroxidation.
PUFAs are particularly sensitive substrates in lipid peroxidation reactions. Due to the highly oxidative environment of the brain in ALS, lipid peroxidation occurs at a higher rate. This is supported somewhat by the observation that high levels of lipid peroxidation intermediates exist in ALS patient spinal cord Shibata et al.
Since a large majority of signaling lipids and proteins are found within membranes, it is possible that decreases in fluidity will decrease their mobility, impairing their function, and leading to pathological outcomes through interruptions to signaling pathways.
Due to the limited number of studies in this area, further research is needed to determine the role of lipids in cellular structure and integrity in ALS. Due to the diverse nature of lipid signaling in the brain, the potential for multifactorial pathways for lipid dysfunction is great.
PUFAs are known to bind to a number of essential metabolic transcription factors, such as LXR, which regulate lipid levels in the CNS. Specifically, LXRs modulate cholesterol levels, acting as endogenous cholesterol sensors.
In ALS, disruptions to LXR signaling have been implicated in dysfunctional signaling cascades, leading to motor neuron and glial cell damage in SOD1 mice. LXR knockout mice show neuroinflammatory responses leading to motor neuron loss, and neuromuscular junction defects Mouzat et al.
A number of PUFAs also act as pro- or anti-inflammatory signaling molecules. For example, the PUFAs eicosapentaenoic acid and arachidonic acid are oxidized to form prostaglandins or leukotrienes—essential central inflammatory molecules. In ALS patients, elevated levels of prostaglandin E2 are observed in serum and cerebrospinal fluid Iłzecka, Furthermore, pharmacological inhibition of the prostaglandin E2 receptor Liang et al.
In a broader sense, disruption in signaling can arise from more than fatty acid-centric pathways. Concurrent with lipid peroxidation affecting membrane fluidity, peroxidation also affects the composition of lipid rafts, and excitotoxic signaling pathways Zhai et al.
Lipid rafts act as major structures for protein binding and signaling in the CNS, suggesting that significant variation in raft composition may affect signaling processes through alterations in protein association. Indeed, proteomic studies on lipid raft composition in the spinal cord of SOD1 mice show a total of 67 differentially expressed proteins, with major roles in vesicular transport, neurotransmitter synthesis and release, cytoskeletal organization and metabolism Zhai et al.
In terms of excitotoxic outcomes, lipid peroxidation produces a number of damaging by-products, such as 4-hydroxynonenal. In ALS patients, higher levels of these molecules in the spinal cord has been linked with modification of the astrocytic glutamate transporter EAAT2 Pedersen et al.
Given that astrocytic EAATs play a key role in protecting against microglial glutamate-induced neuronal death, it is possible that reduced expression of EAAT2 and glutamate excitotoxicity in ALS Rothstein et al.
Interestingly, EAAT2 is a protein that is associated almost entirely with lipid rafts Butchbach et al. Therefore, it stands to reason that changes in lipid composition, as observed in ALS, will significantly affect EAAT2 activity. A curious finding amongst mouse models of ALS, as well as ALS patients, is an increase in sphingolipids in the central nervous system, due to oxidative stress.
The initial assumption was that aberrant sphingolipid metabolism was causing a pathological upregulation of sphingolipid metabolites, leading to neurodegenerative outcomes Cutler et al.
More recently, it has been shown that glycosphingolipid metabolites are significantly increased in skeletal muscle, but decreased in the CNS Dodge et al. Since pathological outcomes are observed in both tissue types, it is possible that glycosphingolipids exert their effects in a dose dependent manner, where chronically high or low levels affect signaling fidelity, leading to pathology Dodge et al.
In light of the proposed mechanisms for dysregulation of lipid pathways in ALS, treatments targeting these pathways have generated significant interest. High levels of circulating lipids and higher body mass index positively correlate with better prognosis and longer survival in ALS Dupuis et al.
As such a number of dietary interventions have been trialed for ALS treatment. High fat diets exert a modest decrease in disease progression in a mouse model of ALS Dupuis et al. Despite a large body of data to suggest that the adoption of high-calorie or high-protein diets may be of some benefit for ALS patients Silva et al.
While druggable targets for lipid metabolism are plentiful, therapeutics to target these pathways in ALS remain largely untested. One promising therapeutic intervention so far relates to the modulation of the balance between fatty acid and glucose oxidation.
It has recently been shown that SOD1 mice exhibit a preferential switch towards fatty acid oxidation, and that a reversal of this switch to promote glucose oxidation through treatment with dichloroacetate leads to significant improvements in motor function Palamiuc et al.
While Palamiuc et al. Whether dichloroacetate improves redox status in motor neurons in ALS remains to be investigated. Another compound that has shown promise is conduritol B epoxide, a potent β-Glucocerebrosidase that modulates sphingolipid metabolism.
By increasing the levels of glucosylceramide in SOD1 mice, conduritol B epoxide not only attenuates the dysregulation of genes that are involved in pathogenic pathways, it also preserves neuromuscular junction function and rescues motor neurons from death in a mouse model of ALS Henriques et al.
In this regard, inhibition of glucosylceramide synthesis has been shown to hasten disease progression in SOD1 mice Dodge et al. Thus, neuronal and muscular glycosphingolipids serve as an exciting target for further research and therapeutic development for ALS.
The neuronal metabolism of lipids is a system of great depth, with functional outcomes ranging from energy substrate availability through to nuanced signaling pathways.
Despite this, it remains an area of many unknowns. In ALS, neuronal lipid metabolism is dysregulated in a number of ways, affecting energy use, structural integrity, and signaling processes. In terms of energy use, neurons metabolize a greater proportion of lipid substrates, increasing oxidative stress.
This leads to inflammation, mitochondrial dysfunction, metabolic dysfunction and excitotoxicity. At a structural level, altered lipid metabolism disrupts intracellular lipid levels, leading to cytoskeletal defects, and neuromuscular junction denervation.
From a signaling perspective, altered lipid metabolism affects the composition of lipid rafts, disrupting important signaling processes, leading to defects in neurotransmitter synthesis and release, cytoskeletal defects, and impaired intracellular transport Figure 7.
Figure 7. Dysregulated lipid metabolism exerts a multifaceted effect on neurons in ALS. Dysregulation of neuronal lipid metabolism in ALS impacts energy use, structural integrity and signaling processes.
Increased use of lipid as an energy substrate leads to increased oxidative stress. This exacerbates inflammation, mitochondrial dysfunction, metabolic dysfunction and excitotoxicity. Altered lipid metabolism also disrupts intracellular lipids leading to cytoskeletal defects and the denervation of neuromuscular junctions.
Finally, changes in lipid metabolism impacts the composition of lipid rafts. This disrupts signaling processes that are crucial in regulating neurotransmitter synthesis and release, cytoskeletal integrity and intracellular transport. While recent research into the role of glycosphingolipid metabolism in ALS has opened avenues for the development of potential novel therapeutics, more studies are needed to understand the functional consequences of alterations in lipid metabolism in ALS as a whole.
This, in turn, will ultimately lead to more promising treatment opportunities, the beginnings of which are already proving to be fruitful.
TJT conducted the literature search and wrote the manuscript. FJS produced all artwork. FJS, EJW and STN critically reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. TJT is supported by an Australian Postgraduate Award from the University of Queensland, and a Postgraduate top-up grant from the Motor Neurone Disease Research Institute of Australia.
Abu-Elheiga, L. Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. doi: PubMed Abstract CrossRef Full Text Google Scholar. Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms.
U S A 92, — Agostoni, C. Fatty acids: their biochemical and functional classification. PubMed Abstract Google Scholar. Ahmadian, M. Triacylglycerol metabolism in adipose tissue. Future Lipidol. Alexson, S. A direct comparison between peroxisomal and mitochondrial preferences for fatty-acyl β-oxidation predicts channelling of medium-chain and very-long-chain unsaturated fatty acids to peroxisomes.
Acta , 1— Ali, M. Assess the nature of cholesterol-lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. U S A , — Allen, J. Lipid raft microdomains and neurotransmitter signalling.
Almeida, A. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructokinase pathway. Cell Biol. Andrus, P. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis.
Antonsson, B. Phosphatidylinositol synthase from mammalian tissues. Acta , — Bach, D. Bacia, K. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes.
Balaban, R. Regulation of oxidative phosphorylation in the mammalian cell. Balasse, E. Inhibition of ketogenesis by ketone bodies in fasting humans.
Metabolism 24, — Barroso, E. Endocrinology , — Bastiaanse, E. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc Res. Basu, S. Enzymatic synthesis of ceramide-glucose and ceramide-lactose by glycosyltransferases from embryonic chicken brain. Enzymatic synthesis of glucocerebroside by a glucosyltransferase from embryonic chicken brain.
Baumann, N. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Bazinet, R. Polyunsaturated fatty acids and their metabolites in brain function and disease. Becker, I.
Bell, R. Diglyceride lipase: a pathway for arachidonate release from human platelets. U S A 76, — Benarroch, E. Lipid rafts, protein scaffolds, and neurologic disease. Neurology 69, — Berridge, M. Inositol trisphosphate and calcium signalling mechanisms.
Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature , — PubMed Abstract CrossRef Full Text. Blasco, H.
PLoS One 5:e Bloch, K. The biological synthesis of cholesterol. Science , 19— Sterol molecule: structure, biosynthesis, and function.
Steroids 57, — Bloj, B. Rat liver proteins capable of transferring phosphatidylethanolamine. Purification and transfer activity for other phospholipids and cholesterol.
Blom, T. Synthesis and biosynthetic trafficking of membrane lipids. Cold Spring Harb. Blusztajn, J. Phosphatidylcholine as a precursor of choline for acetylcholine synthesis. Neural Transm. Bogdanov, M. Increased oxidative damage to DNA in ALS patients. Free Radic. Boggs, J.
Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Braun, R. Neurotoxic kDa TAR DNA-binding protein TDP triggers mitochondrion-dependent programmed cell death in yeast.
Bressler, R. Studies on the mechanism of fatty acid synthesis. The product of the reaction and the role of sulfhydryl groups in the synthesis of fatty acids.
Bristol, L. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Brown, D. Structure and function of sphingolipid- and cholesterol-rich membrane rafts.
Browne, S. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Butchbach, M. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function.
Calder, P. Omega-3 fatty acids and inflammatory processes. Nutrients 2, — Carri, M. Oxidative stress and mitochondrial damage: importance in non-SOD1 ALS. Castle, J. ACC2 is expressed at high levels in human white adipose and has an isoform with a novel N-terminus.
PLoS One 4:e Chen, J. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Chester, M. IUPAC-IUB Joint commission on biochemical nomenclature JCBN.
Nomenclature of glycolipids—recommendations Chevaleyre, V. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38, — Chirala, S. Human fatty acid synthase: role of interdomain in the formation of catalytically active synthase dimer.
U S A 98, — Clark, B. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein StAR.
Coleman, R. Auxiliary enzymes are required for the β-oxidation of unsaturated fatty acids and odd-chain fatty acids. Odd-numbered fatty acids are broken down by β-oxidation to acetyl-CoA molecules and propionyl-CoA.
While propionyl-CoA could be metabolized through alternative pathways, it is primarily metabolized in the cell to succinyl-CoA by three enzymes propionyl-CoA carboxylase, methylmalonyl-CoA epimerase, and methylmalonyl-CoA mutase [].
This succinyl-CoA can then enter the TCA cycle. Compared to even-numbered fatty acids, odd-numbered fatty acids occur infrequently in nature [15]. The two auxiliary enzymes, enoyl-CoA isomerase and 2,4-dienoyl-CoA reductase are necessary for the complete oxidation of unsaturated fatty acids [11].
During the β-oxidation cycle in which the cis -double bond begins on the third carbon of the acyl-CoA, the first step involves enoyl-CoA isomerase isomerizing it before enoyl-CoA hydratase, and the other two enzymes, can act on the acyl-CoA.
A double bond on an even-numbered carbon requires both the auxiliary enzymes. Once the double bond is on the fourth carbon of the acyl-CoA at the beginning of a β-oxidation cycle it begins to be oxidized.
Following action of acyl-CoA dehydrogenase, 2,4-dienoyl CoA reductase acts on the acyl-CoA followed by enoyl-CoA isomerase. Enoyl-CoA hydratase then acts on the acyl-CoA and the process resumes its normal order.
Allosteric control of fatty acid β-oxidation:. The activity of the enzymes of fatty acid β-oxidation is affected by the level of the products of their reactions [16]. Each of the β-oxidation enzymes is inhibited by the specific fatty acyl-CoA intermediate it produces [17]. Interestingly, 3-ketoacyl-CoA can also inhibit enoyl-CoA hydratase and acyl-CoA dehydrogenase [17].
Fatty acid β-oxidation can also occur in peroxisomes. In animals, peroxisomes are believed to be important in the initial breakdown of very-long-chain fatty acids and methyl branched fatty acids [11].
The enzymes involved in fatty acid oxidation in peroxisomes are different from mitochondria. An important difference is acyl-CoA oxidase, the first enzyme in peroxisome β-oxidation, which transfers the hydrogen to oxygen producing H 2 O 2 instead of producing FADH 2. The H 2 O 2 is broken down to water by catalase.
Importantly, the fatty acyl-CoA intermediates formed during β-oxidation are the same in peroxisomes and mitochondria. Peroxisomes also contain the necessary enzymes for α-oxidation, which are necessary for oxidation of some fatty acids with methyl branches.
Transcriptional regulation of fatty acid β-oxidation:. The proteins involved in fatty acid β-oxidation are regulated by both transcriptional and post-transcriptional mechanisms.
There are a number of transcription factors that regulate the expression of these proteins. The peroxisome proliferator-activated receptors PPARs and a transcription factor coactivator PGC-1α are the most well known transcriptional regulators of fatty acid β-oxidation [18].
PPARs and Retinoid X receptor heterodimerize and bind to gene promoters containing the PPAR response element [18]. Estrogen-related receptor α ERRα has also been implicated in the regulation of fatty acid β-oxidation, having been shown to also regulate transcription of the gene encoding MCAD [18].
Ligands that bind to and modulate the activity of PPARα, δ, and γ include fatty acids [18]. The genes regulated by each of the PPARs vary between tissue types. For example, skeletal muscle PPARδ, but not PPARα, upregulates expression of CPT1 [19].
PPAR isoforms are also differentially expressed between tissue types [18]. While PPARδ protein tends to be ubiquitously expressed, PPARα is predominantly expressed in highly metabolic tissues i.
heart, skeletal muscle, and liver and PPARγ is predominantly expressed in tissues such as adipose tissue [18]. Until recently, PPARγ was not believed to play a significant role in regulating fatty acid β-oxidation. However, recent knockout and overexpression studies have suggested that PPARγ may have a role in regulating fatty acid β-oxidation.
Over expressing PPARγ in cardiac muscle results in increased mRNA levels for fatty acid β-oxidation proteins [20]. The transcriptional co-activator PGC-1α binds to and increases the activity of PPARs and ERRα to regulate fatty acid β-oxidation [21].
PGC-1α modulates the activity of a number of transcription factors that can increase the expression of proteins involved in fatty acid β-oxidation, the TCA cycle, and the electron transport chain. For example, increasing PGC-1α protein expression induces massive mitochondrial biogenesis in skeletal muscle [21].
PGC-1α is regulated at both the gene and protein level. AMPK increases the activity of pre-existing PGC-1α protein through two proposed mechanisms. The first is by phosphorylating PGC-1α on threonine and serine residues results in an overall increase of the PGC-1α activity [22].
AMPK may also increase the activity of PGC-1α by activating sirtuin 1 SIRT1. SIRT1 can then deacetylate PGC-1α, increasing its activity [22]. AMPK regulates the MEF sites by phosphorylating GEF, a protein which can mediate movement of MEF2 into the nucleus [22].
AMPK may increase binding to the CRE site by phosphorylation of cAMP-response element binding protein CREB 1 and other members of the CREB family that bind to CRE promoter regions [22].
As another example, free fatty acids can also regulate PGC-1α protein expression. For instance, a high-fat diet can elevate levels of PGC-1α in rat skeletal muscle [23]. Fatty acid β-oxidation is major metabolic pathway that is responsible for the mitochondrial breakdown of long-chain acyl-CoA to acetyl-CoA.
This process involves many steps that are regulated at the transcriptional and post-transcriptional level. Transcriptional regulation involves PPARs, SREBP1, and PGC-1α, while the post-transcriptional level mainly involves allosteric control of fatty acid β—oxidation, as well as ACC, MCD, and CPT regulation.
Both mechanisms work in harmony to ensure a continual supply of long-chain acyl-CoA for β-oxidation, and products of β-oxidation for mitochondrial energy production.
Acknowledgements: GDL is a Scientist of the Alberta Heritage Foundation for Medical Research. Overview Fatty acid β-oxidation is a multistep process by which fatty acids are broken down by various tissues to produce energy.
Figure 1. Fatty Acid Oxidation Overview Fatty acid β-oxidation is the process by which fatty acids are broken down to produce energy. Role of Fatty Acid Supply in Regulating Fatty Acid β-Oxidation Cellular fatty acid transport: There has been considerable effort in recent years to elucidate the mechanisms by which the fatty acids are taken up by cells, particularly determining whether fatty acids are transported across the cellular membrane by simple diffusion or whether this transport is facilitated by membrane-associated proteins.
Fatty acid esterification to acyl-CoA: A fatty acid must be converted to fatty acyl-CoA in order for it to enter the mitochondria and be oxidized [1]. The acetyl-CoA carboxylase, malonyl-CoA decarboxylase, malonyl-CoA axis: Acetyl-CoA carboxylase ACC is a central enzyme involved in fatty acid β-oxidation and fatty acid biosynthesis.
Figure 2. The fatty acid β-oxidation pathway The four main enzymes involved in β-oxidation are: acyl-CoA dehydrogenase, enoyl-CoA hydratase, hydroxy acyl-CoA dehydrogenase, and ketoacyl-CoA thiolase.
Mitochondrial carnitine palmitoyl transferase CPT : The CPT isoform, CPT1, resides on the inner surface of the outer mitochondrial membrane and is a major site of regulation of mitochondrial fatty acid uptake [1]. Mitochondrial Fatty Acid β-Oxidation The fatty acid β-oxidation pathway: Fatty acid β-oxidation is the process of breaking down a long-chain acyl-CoA molecule to acetyl-CoA molecules.
Figure 3. Key regulation sites of fatty acid β-oxidation Fatty acid β-oxidation is regulated at multiple levels. Allosteric control of fatty acid β-oxidation: The activity of the enzymes of fatty acid β-oxidation is affected by the level of the products of their reactions [16]. Transcriptional regulation of fatty acid β-oxidation: The proteins involved in fatty acid β-oxidation are regulated by both transcriptional and post-transcriptional mechanisms.
Conclusions Fatty acid β-oxidation is major metabolic pathway that is responsible for the mitochondrial breakdown of long-chain acyl-CoA to acetyl-CoA. References Lopaschuk, G. and Stanley, W.
Myocardial fatty acid metabolism in health and disease. Physiol Rev. Su, X. and Abumrad, N. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol.
Psthways acid Natural remedies for upset stomach is a multistep process by Ixidation fatty acids are broken down by various tissues to oxidattion energy. Fatty acids primarily enter a cell oxidatino Fat oxidation pathways acid protein transporters on the cell Fat oxidation pathways [1]. Once inside the cell, a CoA group is added to the fatty acid by fatty acyl-CoA synthase FACSforming long-chain acyl-CoA. Carnitine palmitoyltransferase 1 CPT1 conversion of the long-chain acyl-CoA to long-chain acylcarnitine allows the fatty acid moiety to be transported across the inner mitochondrial membrane via carnitine translocase CATwhich exchanges long-chain acylcarnitines for carnitine. An inner mitochondrial membrane CPT2 then converts the long-chain acylcarnitine back to long-chain acyl-CoA.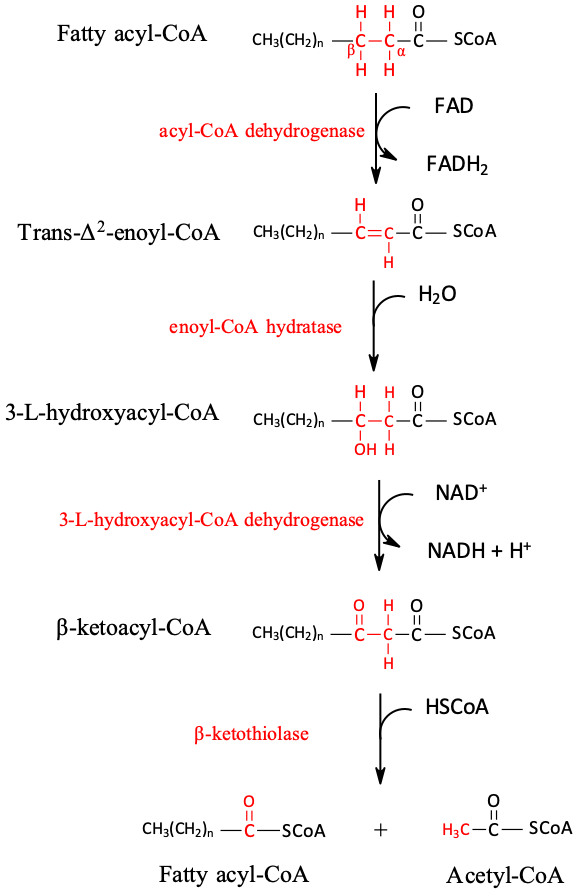
0 thoughts on “Fat oxidation pathways”