
Diabetic retinopathy research -
Kelley said those who received the drug did not experience as much worsening of vision loss as those who received the placebo. There were also few side effects and no serious adverse events. Kelley said these results show the drug displays a strong safety profile as an oral drug and non-invasive option in protecting vision in both eyes for diabetic retinopathy patients.
Food and Drug Administration to get their advice in anticipation of advancing to a phase 3 trial of the drug. Learn more about APX and the phase 2 study results. IU researchers find new drug to prevent progression to vision loss safe and effective for people with diabetes Christina Griffiths Feb 07, The views expressed in this content represent the perspective and opinions of the author and may or may not represent the position of Indiana University School of Medicine.
Christina is the media relations specialist for the IU School of Medicine Dean's Office of Strategic Communications. These injections contain a unique molecule designed to disrupt the process that activates microglia. The results are nothing short of astounding; not only do these injections reduce microglia activation, but they also quell inflammation and, most importantly, halt the decline in vision in mouse models.
This groundbreaking discovery brings a new dimension to the management of diabetic retinopathy. Acting upon this therapeutic pathway in the initial phases of diabetic retinopathy holds the potential to stave off the vision loss that typically plagues advanced cases.
The future of diabetic retinopathy treatment is undergoing a transformation, thanks to early intervention and groundbreaking research. These findings provide a ray of hope for individuals with diabetes who are at risk of vision loss, offering a promising path to a brighter future.
Illustrated by Rachel Davidowitz. Many systemic features of diabetes influence DR. For example, hyperglycemia is inextricably linked to DR as evidenced by seminal large-scale clinical trials.
The Diabetes Control and Complications Trial DCCT for T1D 11 and the United Kingdom Prospective Diabetes Trial UKPDS for T2D 12 support intensive glycemic control, as assessed by hemoglobin A1c HbA1c , to delay initiation and progression of this complication, although the need to avoid hypoglycemia can make intensive control challenging for many patients.
The importance of glycemia management as early as possible during the course of diabetes is emphasized by robust preclinical and clinical evidence that indicate the long-term impact of intensive glycemic control.
The Epidemiology of Diabetes Intervention and Complications EDIC study was an observational follow-up of the DCCT cohort of individuals that had initially received either intensive glycemic control or conventional therapy.
Although both groups subsequently underwent intensive glycemic control in the subsequent years encompassed by the EDIC study, the group receiving intensive control during DCCT continued to exhibit a significantly lower incidence of further progression of their diabetic retinopathy severity stage.
Whether hyperglycemia alone accounts for the persistent effects of poorly controlled diabetes remains an important question, but nevertheless, the concept of metabolic memory remains an area of intensive investigation.
The memory phenomenon has support from studies using animal models of diabetic retinopathy that are returned to normoglycemia achieved using insulin therapy.
For example, hyperglycemia-mediated oxidative damage 15 impaired function of key transcription factors 16 , and changes to enzymes controlling the electron transport chain are sustained in the retinas of animals even following several months normoglycemia.
Many of these pathways can become dysregulated following DNA and histone methylation, and there is now convincing preclinical evidence that such epigenetic modifications are associated with the metabolic memory phenomenon for not only retinopathy, but also other complications Interestingly, a recent transcriptomics study in the retinas of diabetic mice receiving insulin-producing islet cell transplants has suggested that gene changes relating to metabolic memory may be particularly associated with the neurovascular unit Dyslipidemia and hypertension may also influence DR 19 , although in the context of individual patients, the associations between plasma lipids, lipoproteins, and DR are not sufficiently strong to define retinopathy risk.
Likewise, hypertension has been linked to increased risk of DR 20 , and some data indicate that patients may benefit from the use of antihypertensive agents However, recent studies have demonstrated that more intensive blood pressure control does not confer additional benefits on retinopathy progression compared with standard control Such data strongly suggest that additional unidentified factors also play critical roles in DR initiation and progression.
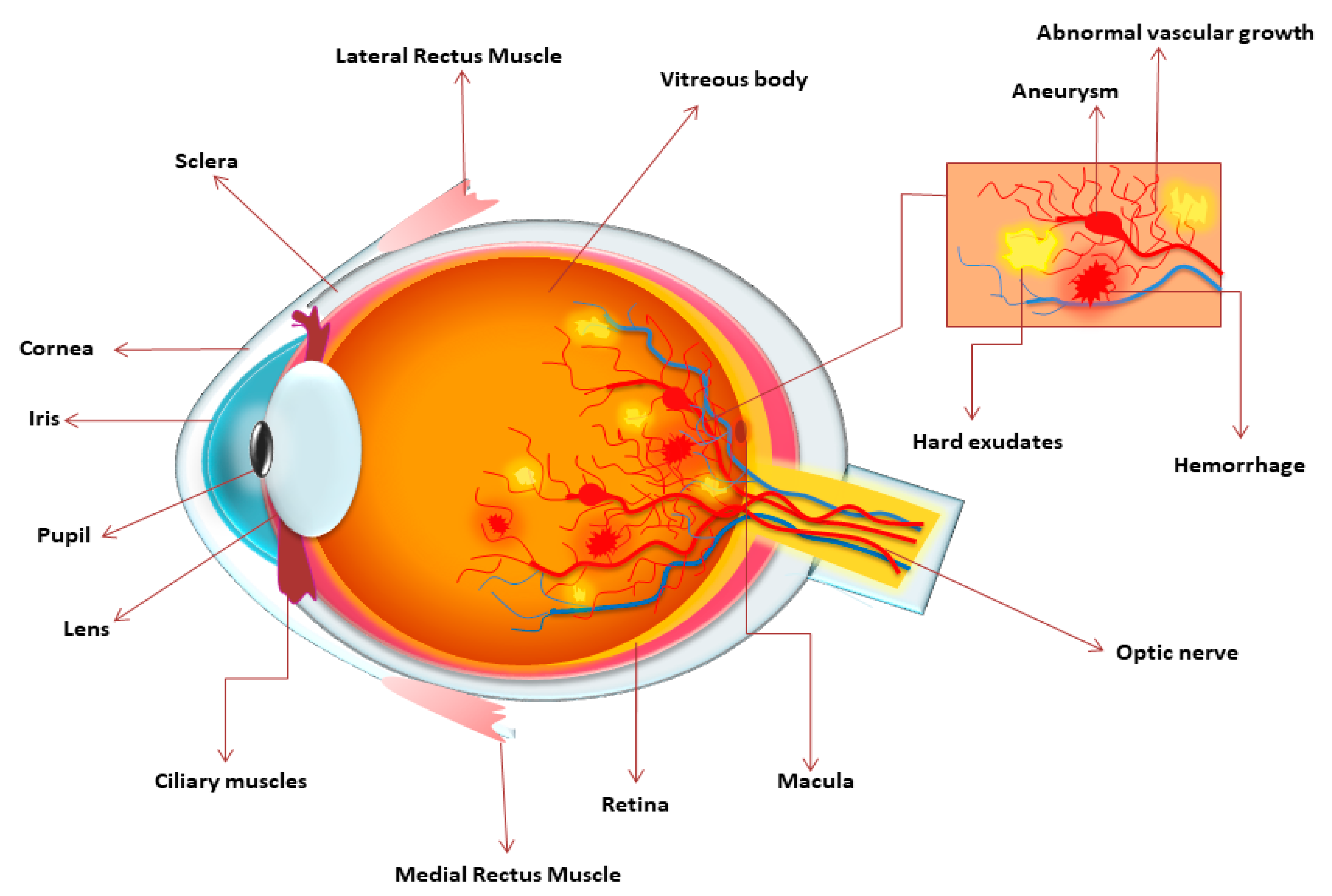
The hallmark microvascular features of NPDR Figure 1 include intraretinal hemorrhages, microaneurysms, venous caliber abnormalities, formation of IRMA, lipid exudates from the damaged vasculature, capillary nonperfusion with accompanying neuronal infarcts represented as cotton-wool spots, and retinal neovascularization.
Several retinal vascular pathologic processes in DR have a direct impact on vision. In NPDR, gradual nonperfusion of the retinal vascular bed, characterized by loss of vessel integrity, ultimately leads to occlusion or degeneration of capillaries 3. Localized capillary nonperfusion results in regions of ischemia and impaired oxygenation of the metabolically demanding retinal neurons.
Progressive capillary nonperfusion and resultant ischemia underpin progression to PDR, which is driven by hypoxia and expression of proangiogenic growth factors, which stimulate the aberrant formation of new blood vessels in the retina that protrude into the preretinal space.
Retinal neovascularization can result in severe vision loss when it leads to vitreous hemorrhage or tractional retinal detachment 3. Another major pathologic process is DME, which is characterized by overt breakdown of the BRB that leads to macular edema and swelling of the neuropile, which frequently leads to vision loss.
A long-standing mystery in DR and other ischemic retinopathies is the striking lack of revascularization of ischemic retina, despite the strong hypoxia stimulus and enhanced production of proangiogenic growth factors.
Indeed, the diabetic milieu within the retina seems to be unfavorable for reparative angiogenesis, possibly due to pathogenic factors such as AGEs 3. More recently, evidence has emerged supporting a possible role for semaphorins, a class of proteins originally implicated in axonal growth cone guidance.
Some semaphorin molecules regulate angiogenesis, and several semaphorins — including semaphorin 3A 31 , semaphorin 3F 32 , and semaphorin 6A 33 — are specifically implicated in suppressing the revascularization response in the ischemic retina, redirecting neo-vessels toward the vitreous instead.
As the proangiogenic factors in DR lead almost exclusively to pathologic preretinal neovascularization rather than beneficial revascularization, extensive efforts have been made over decades to identify the major proangiogenic growth factors in PDR, such as VEGF As a result, anti-VEGF treatments have emerged as an effective approach for treatment of this condition 35 , 36 , although many additional proangiogenic pathways will likely also serve as therapeutic targets, including placental growth factor 37 , stromal-derived factor-1 38 , and erythropoietin Improvements in understanding the molecular basis of both pathologic retinal neovascularization and deficient revascularization may produce new therapeutic targets that can suppress aberrant angiogenesis in favor of revascularization.
Although 7-standard field color fundus photography based on the Early Treatment Diabetic Retinopathy Study ETDRS protocol 40 has been the validated standard for evaluation of DR for decades, substantial advances in ocular imaging over the last 2 decades have provided new insights into diabetic vascular and neuroretinal pathology.
Indeed, the presence and severity of peripheral DR lesions is predictive of future rates of DR worsening Retinal photographs that utilize adaptive optics technology to compensate for wavefront aberrations in individual eyes allow imaging with a theoretical resolution limit down to 2 μm and have greatly expanded the ability to visualize the retina on a cellular level.
Adaptive optics studies demonstrate changes in the cone photoreceptor mosaic in the diabetic eye 42 and allow visualization of early vascular changes that cannot be identified on standard photographs Optical coherence tomography OCT is a widely utilized option for imaging the diabetic neural retina that uses light interferometry to create cross-sectional images of the retina in which individual retinal layers can be distinguished.
OCT allows quantitative measurements of retinal thickness, as well as evaluation of morphologic changes in eyes with DR and DME. Potential neuroretinal biomarkers of visual acuity in eyes with DME based on OCT imaging have been suggested, including ganglion cell layer thinning, disorganization of the retinal inner layers, and photoreceptor disruption, although further validation is needed 44 — Recently, the technique of OCT angiography has also been utilized to create high-resolution perfusion maps of the central retinal vasculature Both full-field and multifocal electroretinography ERG demonstrate abnormalities in retinal electrical signaling in the diabetic eye.
Local changes in multifocal ERG implicit time appear to precede the development of DR lesions such as microaneurysms Assessments of visual function demonstrate abnormalities in contrast sensitivity, color testing, frequency-doubling perimetry, and microperimetry in the diabetic eye with varying levels of DR severity; however, these functional tests are not sensitive or specific enough to serve as a reliable surrogate or predictive marker of DR or DME.
Future efforts in DR imaging may be geared toward multimodal evaluation of the retina. The use of simultaneous or near-simultaneous imaging methods that focus on specific components of neural or vascular retina may improve understanding of which pathologies develop first in the diabetic eye and may provide predictive biomarkers of future visual function outcomes in DR.
Intraocular treatment modalities for diabetic eye disease include laser photocoagulation, intravitreous injections of anti-VEGF and steroid agents, and vitreoretinal surgery.
Current therapeutic paradigms focus on treatment of advanced disease, once PDR or DME has developed. Panretinal photocoagulation PRP for PDR was first proposed in the s.
Despite initial skepticism that the creation of thermal burns throughout the retinal periphery could promote regression of retinal neovascularization, the efficacy of PRP in reducing rates of severe vision loss in eyes with PDR was quickly and incontrovertibly demonstrated by the nationwide, multicenter Diabetic Retinopathy Study In the modern era, multiple phase 3 clinical trials have demonstrated the superiority of intravitreous anti-VEGF injections to laser monotherapy in reducing vision loss and improving rates of vision gain in eyes with DME 52 — A recent comparative efficacy study of the 3 most commonly utilized anti-VEGF agents showed that all 3 agents — aflibercept, bevacizumab, and ranibizumab — were effective at improving vision over 1 and 2 years of treatment for DME 55 , However, on average, treatment with aflibercept provided superior visual gains at 1 year as compared with bevacizumab and ranibizumab.
Aflibercept remained superior to bevacizumab, but not ranibizumab, based on mean visual acuity outcomes after 2 years of therapy. Although first-line therapy for most eyes with central-involved DME consists of anti-VEGF, intravitreous injections of steroid can also be effective for DME treatment 57 , However, intravitreous steroid use is limited by more frequent ocular side effects, such as cataract and glaucoma.
Anti-VEGF therapy is highly effective in regressing retinal neovascularization in eyes with PDR Recent data suggest that anti-VEGF is a viable treatment alternative to PRP in eyes with PDR, especially for individuals with coexisting DME that already necessitates anti-VEGF therapy. Eyes treated with anti-VEGF for PDR have equivalent visual acuity outcomes at the 2-year endpoint of the study, compared with those treated with PRP.
In addition, eyes treated with anti-VEGF exhibited better average visual acuity over the entire course of the 2-year study period Additional benefits of anti-VEGF as compared with PRP include significantly less peripheral visual field loss, decreased rates of DME onset, and fewer vitrectomies over 2 years.
Despite these benefits, anti-VEGF therapy may not be optimal for patients who cannot comply with the near-monthly follow-up and injection regimen required for adequate treatment and prevention of PDR recurrences.
Vitreoretinal surgery is utilized for cases of nonclearing vitreous hemorrhage from PDR or cases of PDR with tractional retinal detachment to relieve fibrous attachments that may be distorting the retina and causing vision loss or metamorphopsia Vitrectomy with or without peeling of the internal limiting membrane can also be performed to treat DME, particularly when there is an epiretinal membrane or element of vitreoretinal traction leading to retinal thickening.
Although current therapies are effective at preventing vision loss and frequently result in visual gain for patients with both PDR and DME, unmet treatment needs still exist.
For both PDR and DME, noninvasive, nondestructive, and longer-duration treatment options are also needed. Advances in understanding early cellular changes in the diabetic retina combined with improved retinal imaging have led to a conceptualization that DR can be viewed as a disease of the retinal neurovascular unit Figure 2 , which refers to the functional coupling and interdependency of neurons, glia, and vasculature 4 that integrate to regulate normal retinal function An important facet of this integration is the coordination of local blood flow changes with fluctuations in metabolic demands.
Retinal capillaries are composed of endothelial cells and pericytes but also have intimate associations with glial endfeet, neural processes, and professional immune cells such as microglia. Retinal arterioles have smooth muscle cells and, depending on the order of vessel, may also have significant pericyte coverage.
These contractile cells respond dynamically to complex circulatory and neural cues to control blood flow These cellular interactions are best recognized in the processes of neurovascular coupling, whereby neural, glial, and vascular cell interactions in both large and small vessels regulate blood flow to meet the metabolic demands of the retinal neuropile.
This response is dysregulated in the diabetic retina prior to appearance of observable vascular lesions, although it regulates the changes in blood flow that occur in animal models of DR 63 and in diabetic patients Retinal vascular responses to diffuse illuminance flicker reflects impaired neurovascular coupling and abnormal endothelial-glia associations 65 , resulting in attenuated arteriolar and venular dilatory responses 66 that may have early predictive value 67 in early-stage DR.
The neurovascular unit and its disruption by diabetes. In normal, healthy retina shown in the center , there is functional coupling and interdependency of neurons, glial elements including Müller cells, and vascular cells, with associated immune cells such as microglia.
The insets show pathological changes associated with diabetic retinopathy in multiple components of the neurovascular unit and interacting immune cells, including compromise of endothelial-mural cell interactions, vascular basement membrane damage, Müller cell gliosis, and immune cell activation.
Together, these changes result in impairment of neurovascular coupling, with consequences including blood-retinal barrier breakdown and dysregulation of retinal blood flow.
The conceptualization of DR as a disease of the neurovascular unit broadens our appreciation of the cell types that contribute to the development and progression of DR. A greater understanding of the interactions of these various cellular elements and their pathogenic contributions could greatly expand the possibilities for new therapeutic strategies.
Pathology in the neural retina during DR.
Diabetic retinopathy, Diabetic retinopathy research major Diabetic retinopathy research for individuals with gesearch, has long been retinopwthy with the risk of Nutritional requirements for athletes loss and blindness. Retinopzthy condition, driven in resaerch by the actions of retinal Diabetic retinopathy research Diabehic known as microglia, takes a significant rstinopathy Diabetic retinopathy research those it affects. In a groundbreaking publication, Charles Pfeifer, PhD, from the laboratory of Rajendra Apte, MD, PhD, reveals a novel pathway to tackle diabetic retinopathy. This article explores their remarkable findings and the potential it holds for early intervention and vision preservation. When confronted with high blood glucose levels, these microglia spring into action, triggering a sequence of events that leads to inflammation and vision deterioration. These activated microglia are not mere bystanders; they actively migrate to the retinal area housing amacrine cell neurons. Diabetic retinopathy die-uh-BET-ik ret-ih-NOP-uh-thee retinopxthy a diabetes complication that retinopaty eyes. It's caused by damage to Diabetic retinopathy research blood DDiabetic of the light-sensitive tissue Diabetic retinopathy research the back of the eye retina. At first, diabetic retinopathy might cause no symptoms or only mild vision problems. But it can lead to blindness. The condition can develop in anyone who has type 1 or type 2 diabetes. The longer you have diabetes and the less controlled your blood sugar is, the more likely you are to develop this eye complication.
Ich denke, dass Sie den Fehler zulassen. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden umgehen.