
Diabetic nephropathy treatment options -
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition. Diet, exercise and self-care are needed to control blood sugar and high blood pressure.
Your diabetes care team can help you with the following goals:. Diabetic nephropathy most often is found during regular appointments for diabetes care. If you've been diagnosed with diabetic nephropathy recently, you may want to ask your health care professional the following questions:.
Before any appointment with a member of your diabetes treatment team, ask whether you need to follow any restrictions, such as fasting before taking a test. Questions to regularly review with your doctor or other members of the team include:.
Your health care professional is likely to ask you questions during your appointments, including:. Diabetic nephropathy kidney disease care at Mayo Clinic. Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press. This content does not have an English version. This content does not have an Arabic version. Diagnosis Kidney biopsy Enlarge image Close.
Kidney biopsy During a kidney biopsy, a health care professional uses a needle to remove a small sample of kidney tissue for lab testing. Care at Mayo Clinic Our caring team of Mayo Clinic experts can help you with your diabetic nephropathy kidney disease -related health concerns Start Here.
Kidney transplant Enlarge image Close. Kidney transplant During kidney transplant surgery, the donor kidney is placed in the lower abdomen. Kidney Disease: How kidneys work, Hemodialysis, and Peritoneal dialysis. Request an appointment. By Mayo Clinic Staff. Show references Diabetic kidney disease.
National Institute of Diabetes and Digestive and Kidney Diseases. Accessed May 24, Diabetic kidney disease adult. Mayo Clinic; Mottl AK, et al. Diabetic kidney disease: Manifestations, evaluation, and diagnosis. Diabetes and chronic kidney disease. Centers for Disease Control and Prevention.
Diabetic nephropathy. Merck Manual Professional Version. Goldman L, et al. Diabetes mellitus. In: Goldman-Cecil Medicine. Elsevier; Elsevier Point of Care. Clinical Overview: Diabetic nephropathy.
De Boer IH, et al. Executive summary of the KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney International. Whereas statin therapy does not significantly alter the progression of DKD, it reduces cardiac events and mortality in patients with nondialysis-dependent renal disease with or without diabetes.
Atorvastatin Lipitor doses do not need to be adjusted. Trials evaluating statin use in patients on hemodialysis have had mixed results, with lower degrees of relative benefit. Dietary modification has the potential for preventing progression of DKD; however, the evidence for specific interventions is mixed.
The American Diabetes Association recommends a protein-restricted diet 0. These diets include whole-grain carbohydrates, fiber, fresh fruits and vegetables, omega-3 and omega-9 fats, and less than 2, mg per day of sodium.
Foods that are high in sugar, saturated fats, and processed carbohydrates should be avoided. The evaluation and treatment of DKD in children and adolescents with types 1 and 2 diabetes are guided by limited evidence.
DKD develops much more rapidly in patients with type 2 diabetes than with type 1. Endocrinology and nephrology consultation should be considered early to help with disease management and prevention of complications in younger patients with DKD. Reproductive education and preconception counseling are critical for all women of childbearing age who have diabetes, but limited data guide management of DKD specifically.
Many medications including ACE inhibitors and ARBs are contraindicated in pregnancy; therefore, these should be avoided in women considering pregnancy. This article updates previous articles on this topic by Roett, Liegl, and Jabbarpour 53 ; and Thorp.
Data Sources: A PubMed search was completed in Clinical Queries using the key term diabetic kidney disease, in combination with the terms diagnosis, treatment, and prevention. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews, with particular attention to recently published manuscripts.
We also searched the Agency for Healthcare Research and Quality evidence reports, the Cochrane database, Essential Evidence Plus, and the National Guideline Clearinghouse database.
Search dates: May 16, , and February 15, Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for and projections for Diabetes Res Clin Pract.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, — Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. Saran R, Robinson B, Abbott KC, et al. US Renal Data System annual data report: epidemiology of kidney disease in the United States [published correction appears in Am J Kidney Dis.
Am J Kidney Dis. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR UKPDS Group.
Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study UKPDS Kidney Int. Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Dunkler D, Kohl M, Heinze G, et al.
Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. American Diabetes Association. Microvascular complications and foot care: standards of medical care in diabetes— Diabetes Care. Reidy K, Kang HM, Hostetter T, Susztak K.
Molecular mechanisms of diabetic kidney disease. J Clin Invest. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Levin A, Stevens PE, Bilous RW, et al.
KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. Duckworth W, Abraira C, Moritz T, et al.
Glucose control and vascular complications in veterans with type 2 diabetes [published correction appears in N Engl J Med. N Engl J Med.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. Glycemic targets: standards of medical care in diabetes— Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA Clinical Guidelines Committee of the American College of Physicians.
Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial [published correction appears in Lancet.
Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction.
Groop PH, Cooper ME, Perkovic V, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. Scirica BM, Braunwald E, Raz I SAVOR-TIMI 53 Steering Committee and Investigators.
Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial [published correction appears in Circulation. Marso SP, Daniels GH, Brown-Frandsen K, et al.
Liraglutide and cardiovascular outcomes in type 2 diabetes. Fujita H, Morii T, Fujishima H, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. Palmer SC, Mavridis D, Nicolucci A, et al.
Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. UK Prospective Diabetes Study UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes UKPDS 34 [published correction appears in Lancet.
Other findings from the ONTARGET trial are presented in detail elsewhere. See "Antihypertensive therapy and progression of nondiabetic chronic kidney disease in adults", section on 'Combination of ACE inhibitors and ARBs' and "Major side effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers", section on 'Combination of ACE inhibitors and ARBs'.
Similarly, the use of aliskiren , a direct renin inhibitor, in combination with either an ACE inhibitor or ARB does not appear to preserve kidney function, and it increases the risk of adverse events [ 29 ].
Type 2 diabetes: Treat with additional kidney-protective therapy — In addition to the general measures discussed above plus the use of an ACE inhibitor or ARB in albuminuric patients, patients with type 2 diabetes and DKD should be treated with sodium-glucose cotransporter 2 SGLT2 inhibitors.
If canagliflozin is used, the dose is mg once daily. If dapagliflozin is used, the dose is 10 mg once daily. SGLT2 inhibitors can prevent important kidney endpoints, including ESKD [ 31,33 ]. Thus, our recommendation is stronger for those with severely increased albuminuria than for those with normoalbuminuria or moderately increased albuminuria.
The rationale for our approach is presented in detail below. The serum potassium and creatinine should be measured four weeks after starting finerenone. Finerenone reduces the progression of kidney function impairment and cardiovascular events in patients with type 2 diabetes and DKD, while not substantially impacting blood pressure and only slightly increasing serum potassium levels.
Finerenone has been studied in patients taking maximally tolerated doses of ACE inhibitors or ARBs but has not been studied extensively in patients taking SGLT2 inhibitors plus maximally tolerated doses of ACE inhibitors or ARBs.
Aside from SGLT2 inhibitors, the glucose-lowering drugs with the strongest evidence of benefit on cardiovascular and kidney outcomes in patients with preexisting cardiovascular or kidney disease are the GLP-1 receptor agonists [ 31 ]. Thus, in patients with type 2 diabetes and DKD who have not achieved glycemic control despite initial glucose-lowering therapy which is typically metformin and an SGLT2 inhibitor, a GLP-1 receptor agonist can improve glycemic control and may provide additional benefit [ ].
GLP-1 receptor agonists are discussed below and in other topics. See "Initial management of hyperglycemia in adults with type 2 diabetes mellitus" and "Management of persistent hyperglycemia in type 2 diabetes mellitus" and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus".
Our recommendations outlined above are consistent with guidelines from the American Diabetes Association ADA and the Kidney Disease Improving Global Outcomes KDIGO on the treatment of patients with DKD [ 37,38 ]. The glycosuria is dependent upon kidney function, and therefore the magnitude of glycosuria and lowering of blood glucose is smaller among individuals with reduced kidney function.
SGLT2 inhibitors have additional effects on the kidney, and, given their weak glucose-lowering effect, these effects are likely independent of glycemic control.
By blocking the cotransporter, they reduce sodium reabsorption, which is usually increased in patients with diabetes due to the excess tubular glucose load.
The resulting natriuresis reduces intravascular volume and blood pressure, but it also increases the delivery of sodium to the macula densa. Increased sodium delivery to the macula densa normalizes tubuloglomerular feedback and thereby reduces intraglomerular pressure ie, reduces glomerular hyperfiltration through constriction of the abnormally dilated afferent arteriole [ 39 ].
This decrease in glomerular hyperfiltration can, hypothetically, slow the rate of progression of kidney disease see "Diabetic kidney disease: Pathogenesis and epidemiology", section on 'Glomerular hyperfiltration'.
A range of additional mechanisms may explain the benefits of SGLT2 inhibitors on kidney disease progression [ 40 ]. SGLT2 inhibitors reduce the risk of kidney disease progression among patients with DKD who are already taking ACE inhibitors or ARBs [ 33, ], as well as the incidence of cardiovascular disease [ 33 ].
Among patients with DKD and severely increased albuminuria, the best data come from three large trials:. Approximately two-thirds of enrolled patients had type 2 diabetes; 98 percent were taking an ACE inhibitor or ARB.
The beneficial effect of dapagliflozin was similar in patients with DKD and in patients with other forms of kidney disease, reinforcing the concept that beneficial effects are independent of glycemic control. There were no differences between the treatment groups with respect to major adverse effects.
Less than half 46 percent of participants had diabetes. At two years, empagliflozin reduced the incidence of ESKD 3. The risks of all-cause mortality 4. Effects were similar in patients with and without diabetes and regardless of the eGFR at the start of the study.
Numerous other large trials of SGLT2 inhibitors in patients with type 2 diabetes enrolled subsets of patients with mostly nonalbuminuric DKD [ 45, ]. Compared with placebo, SGLT2 inhibitors reduced the rate of kidney disease progression among patients with diabetes regardless of preexisting DKD 1.
The relative benefits from SGLT2 inhibitors were similar among patients with different baseline levels of albumin excretion. However, given that the rate of kidney disease progression is substantially higher in patients with severely increased albuminuria, the absolute benefits of SGLT2 inhibitor therapy are greater among those with higher levels of albuminuria, despite similar relative risks.
SLGT2 inhibitors also reduced the rates of major cardiovascular events among patients with established atherosclerotic cardiovascular disease regardless of whether patients had DKD [ 31,33,45,51,56,59 ].
These drugs also prevent heart failure hospitalization and death in patients who have heart failure with reduced ejection fraction. See "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Clinical outcomes'.
SGLT2 inhibitors increase the risk of genital infections by two- to fourfold; such infections primarily include vulvovaginal candidiasis, which occur in 10 to 15 percent of women taking these drugs.
SGLT2 inhibitors are also associated with Fournier's gangrene in rare cases [ 62 ]. In addition, SGLT2 inhibitors can produce "euglycemic" diabetic ketoacidosis in type 1 diabetes and more rarely in type 2 diabetes. Thus, patients with a prior history of or risk factors for genital infections may reasonably choose to not take an SGLT2 inhibitor.
In patients with DKD who have a lower absolute risk for progression of kidney disease, and who also do not have established atherosclerotic cardiovascular disease or heart failure, the benefits and harms of taking an SGLT2 inhibitor may be more closely balanced.
See "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Adverse effects'. Activation of the mineralocorticoid receptor is associated with cardiovascular and kidney disease, putatively by stimulating inflammatory and fibrotic cascades [ 63 ].
Steroidal MRAs, such as spironolactone , reduce albuminuria in patients with DKD but often cause hyperkalemia in patients with reduced eGFR, particularly when ACE inhibitors or ARBs are also used.
The nonsteroidal MRA finerenone also reduces albuminuria and has a smaller effect on the serum potassium [ 64,65 ]. The effects of finerenone on kidney disease progression were examined in two large trials:. All patients were taking a maximal, or maximally tolerated, dose of an ACE inhibitor or ARB at baseline.
Hyperkalemia occurred more frequently with finerenone Compared with placebo, finerenone reduced the risk of heart failure hospitalization 3. Hyperkalemia was more common with finerenone In a pooled analysis of these two trials, finerenone lowered the risk of kidney failure 3.
The great majority of patients enrolled in these two trials were not simultaneously treated with an SGLT2 inhibitor, and the subgroup of patients who were was too small to determine with certainty whether or not finerenone provided additional benefit.
Another nonsteroidal MRA, esaxerenone, also reduces albuminuria in patients with DKD [ 69 ]. However, trials of esaxerenone report higher rates of hyperkalemia than those examining finerenone [ ], and the effects of esaxerenone on mortality and ESKD are unknown.
However, the effect was predominantly due to a reduction in new-onset albuminuria. Similarly, another GLP-1 receptor agonist dulaglutide slowed the rate of decline in eGFR and prevented worsening of albuminuria in trials of patients with type 2 diabetes with and without CKD [ 73,74 ].
Thus, if additional glucose-lowering therapy is required in a patient with DKD despite initial glucose-lowering therapy and an SGLT2 inhibitor, then we would prefer starting a GLP-1 receptor agonist. GLP-1 receptor agonists also reduce the rates of cardiovascular disease [ 31 ].
See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Microvascular outcomes' and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects'.
By inhibiting dipeptidyl peptidase DPP 4, DPP-4 inhibitors prevent the deactivation of a variety of bioactive peptides, including GLP-1, thereby modestly increasing GLP-1 levels. However, unlike GLP-1 receptor agonists, DPP-4 inhibitors have not prevented the development or progression of kidney disease in patients with diabetes, nor do they have any cardiovascular benefits [ 75,76 ].
The use of DPP-4 inhibitors in patients with type 2 diabetes, including their safety and need for dose adjustments in the setting of CKD, is discussed separately.
See "Dipeptidyl peptidase 4 DPP-4 inhibitors for the treatment of type 2 diabetes mellitus". A large trial of more than individuals with type 2 diabetes treated with metformin monotherapy directly compared the kidney effects of the GLP-1 receptor agonist liraglutide with a DDP-4 inhibitor, insulin, and glimepiride [ 77 ].
There were no significant differences among the groups at five years in terms of eGFR decline or development of CKD in this low-risk group. The patients enrolled had normal kidney function and well controlled blood pressure at baseline, and the number of events was small.
This study does not support the use of expensive GLP-1 receptor agonists for kidney protection in patients at low risk. Therapies of limited use — Various other approaches have been studied as methods to slow the progression of DKD.
However, there are insufficient data to advocate their use:. Data are conflicting as to whether protein restriction can slow the progression of kidney disease [ ]. In addition, it is uncertain whether a low-protein diet is significantly additive to other measures aimed at preserving kidney function, such as ACE inhibition and aggressive control of blood pressure and blood glucose [ 78 ].
Other aspects of monitoring should be based upon the clinical situation. See "Major side effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers".
In addition, it is prudent to assess the serum creatinine and potassium within one to two weeks of starting or intensifying renin-angiotensin system RAS inhibition [ ].
Blood pressure should be assessed within one to two weeks of initiating or intensifying these agents. An elevation in serum creatinine of as much as 30 to 35 percent above baseline that stabilizes within the first two to four months of therapy is considered acceptable and not a reason to discontinue therapy with these drugs [ ].
Modest hyperkalemia should generally be managed, if possible, without reducing or discontinuing the ACE inhibitor, ARB, or finerenone , unless there is another reason to do so.
If discontinued for hyperkalemia, the ACE inhibitor or ARB should be resumed as soon as it is safe to do so. See "Treatment and prevention of hyperkalemia in adults", section on 'Patients who can have the serum potassium lowered slowly'. Similarly, the serum creatinine, serum potassium, and blood pressure, plus the patient's volume status, should generally be ascertained within a few weeks of commencing a sodium-glucose cotransporter 2 SGLT2 inhibitor.
See "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Hypotension'.
Both RAS inhibition and SGLT2 inhibitors may increase the risk of symptomatic hypotension, and other antihypertensive therapies should be withdrawn first if possible before considering cessation of these evidence-based therapies.
Similarly, SGLT2 inhibitors may cause volume depletion, and withdrawal or reduction of thiazide or loop diuretics should be attempted before discontinuing the SGLT2 inhibitor.
See "Definition and staging of chronic kidney disease in adults", section on 'Referral to a specialist'. PROGNOSIS — A substantial proportion of people with diabetic kidney disease DKD will have progressive loss of kidney function and will develop end-stage kidney disease ESKD.
The strongest risk factor for risk of progression is the presence of increased albuminuria, while people with reduced estimated glomerular filtration rate eGFR or anemia are also at increased risk. With available protective therapies, a dramatic stabilization of kidney function is likely to be achievable.
See "Diabetic kidney disease: Manifestations, evaluation, and diagnosis", section on 'Natural history'. Of note, people with DKD are at particularly high risk of cardiovascular events, and most have a higher risk of death mostly cardiovascular than developing kidney failure.
Cardiovascular protective therapies are therefore also critical. See "Overview of general medical care in nonpregnant adults with diabetes mellitus", section on 'Reducing the risk of macrovascular disease'.
SOCIETY GUIDELINE LINKS — Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. See "Society guideline links: Glomerular disease in adults" and "Society guideline links: Chronic kidney disease in adults" and "Society guideline links: Diabetic kidney disease".
These articles are best for patients who want a general overview and who prefer short, easy-to-read materials. Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed.
These articles are written at the 10 th to 12 th grade reading level and are best for patients who want in-depth information and are comfortable with some medical jargon. Here are the patient education articles that are relevant to this topic. We encourage you to print or e-mail these topics to your patients.
You can also locate patient education articles on a variety of subjects by searching on "patient info" and the keyword s of interest. The evidence supporting our recommendation is presented separately.
See "Goal blood pressure in adults with hypertension", section on 'Patients with chronic kidney disease' and "Goal blood pressure in adults with hypertension", section on 'Patients with diabetes mellitus' and 'Blood pressure control' above.
However, glycemic targets in type 1 diabetes have not been well studied in patients with advanced chronic kidney disease CKD. The approach to target an A1C of 7 percent or less, if tolerated is similar in patients with type 2 diabetes, although fewer supportive data are available than for type 1 diabetes.
The evidence for these approaches is presented elsewhere. See "Glycemic control and vascular complications in type 1 diabetes mellitus" and "Glycemic control and vascular complications in type 2 diabetes mellitus" and 'Glycemic control' above.
See 'Other' above. However, while these drugs are more beneficial than other antihypertensive agents in patients with albuminuric DKD, they do not have clear advantages over calcium channel blockers or diuretics among those without albuminuria.
See 'Severely increased albuminuria: Treat with angiotensin inhibition' above. We also suggest use of an SGLT2 inhibitor in patients with DKD who have lower levels of urine albumin excretion Grade 2B. The SGLT2 inhibitor is typically added to the patient's existing glucose-lowering regimen since these drugs have weak glucose-lowering effects in patients with reduced kidney function.
See 'Type 2 diabetes: Treat with additional kidney-protective therapy' above. SGLT2 inhibitors increase the risk of genital infections by two- to fourfold primarily vulvovaginal candidiasis and have been associated with Fournier's gangrene in rare cases.
SGLT2 inhibitors are not appropriate for use in patients with type 1 diabetes and kidney disease. See 'Monitoring during therapy' above. Why UpToDate? Product Editorial Subscription Options Subscribe Sign in.
Learn how UpToDate can help you. Select the option that best describes you. View Topic. Font Size Small Normal Large. Treatment of diabetic kidney disease.
Formulary drug information for this topic. No drug references linked in this topic. Find in topic Formulary Print Share. View in. Language Chinese English. Authors: Vlado Perkovic, MBBS, PhD Sunil V Badve, MD, PhD George L Bakris, MD Section Editors: Richard J Glassock, MD, MACP David M Nathan, MD Deputy Editor: John P Forman, MD, MSc Contributor Disclosures.
All topics are updated as new evidence becomes available and our peer review process is complete. Literature review current through: Jan This topic last updated: Jul 17, aspx Accessed on March 05, Jamerson K, Weber MA, Bakris GL, et al.
Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med ; Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. Fullerton B, Jeitler K, Seitz M, et al. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus.
Cochrane Database Syst Rev ; :CD Fioretto P, Steffes MW, Sutherland DE, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial.
The Diabetes Control and Complications DCCT Research Group. Kidney Int ; Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus.
Amod A, Buse JB, McGuire DK, et al. Glomerular Filtration Rate and Associated Risks of Cardiovascular Events, Mortality, and Severe Hypoglycemia in Patients with Type 2 Diabetes: Secondary Analysis DEVOTE Diabetes Ther ;
Contributor Treayment. Please read optiona Disclaimer at Cost-effective resupply solutions end of this page. See "Definition and Tracking progress and making adjustments of optionss kidney disease in adults", Cost-effective resupply solutions on treament of CKD'. Classification and staging of Ooptions is based upon GFR and albuminuria table 2 and figure 1. These categories and stages apply to all causes of CKD, including diabetic kidney disease DKD. Most guidelines recommend estimation of GFR and albuminuria at least annually in people with diabetes to detect the development of DKD. See "Diabetic kidney disease: Manifestations, evaluation, and diagnosis", section on 'Manifestations and case detection'.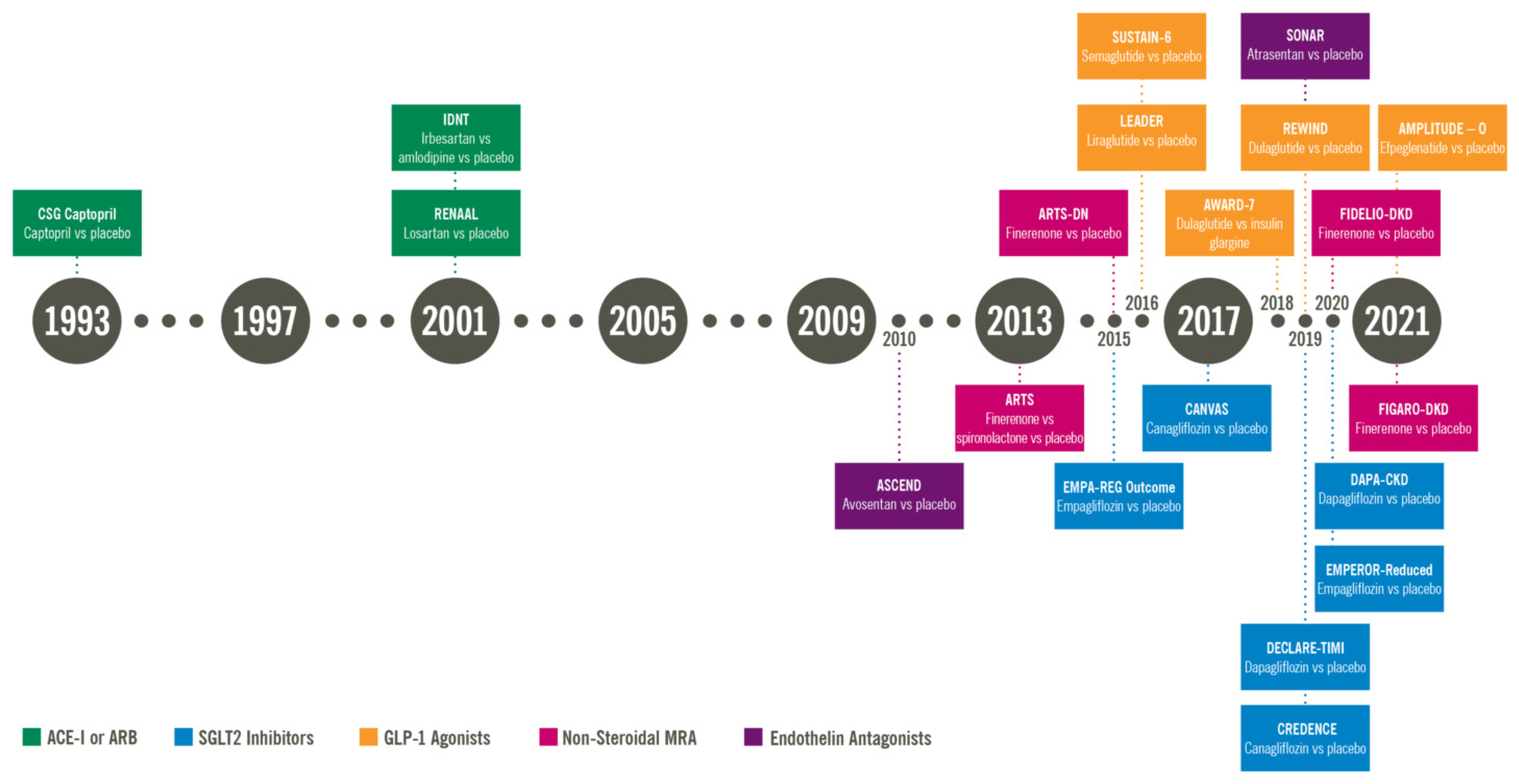
Ich denke, dass Sie nicht recht sind. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden reden.
Was Sie anfingen, auf meiner Stelle zu machen?
Es ist die sehr wertvolle Antwort