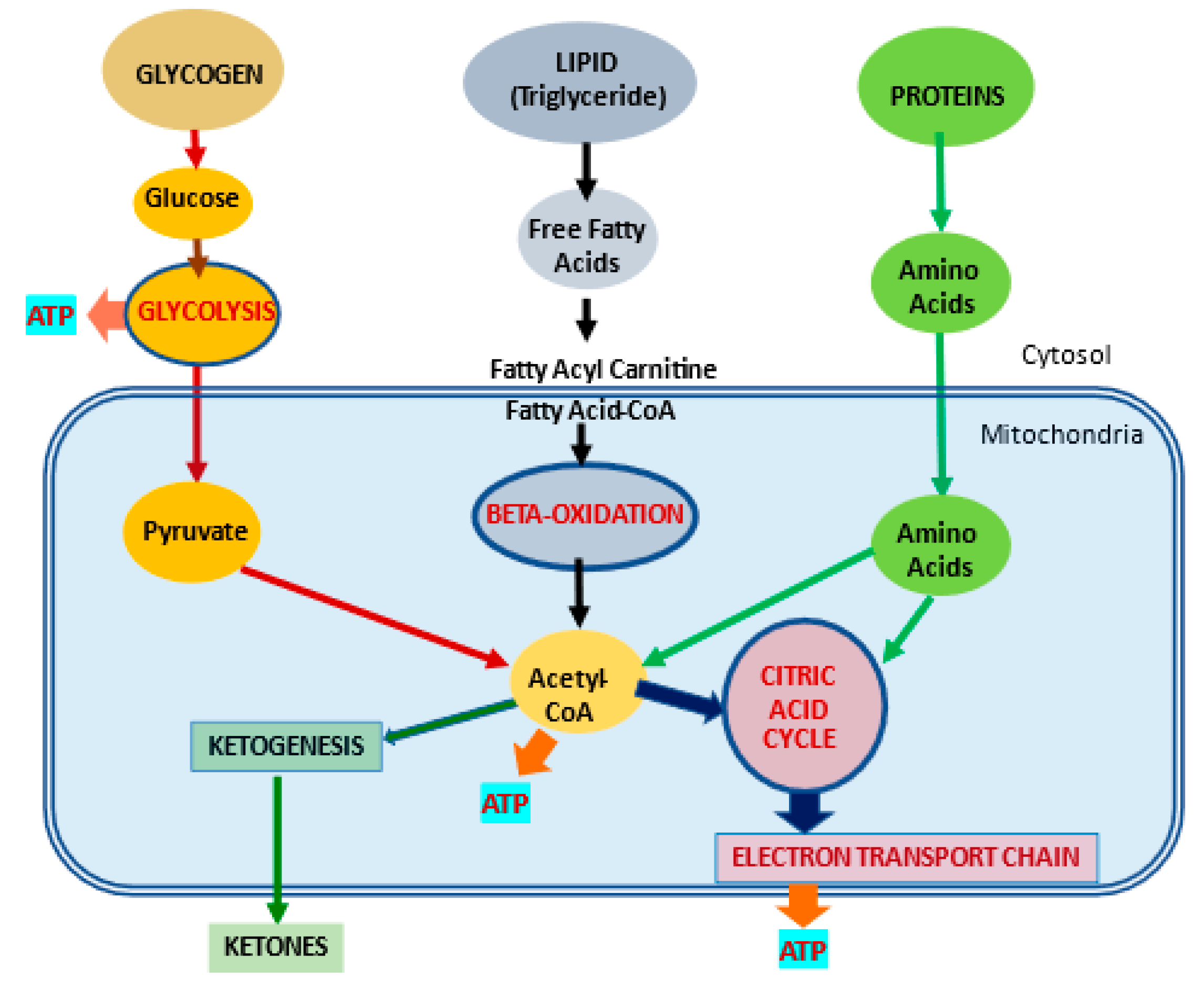
L-carnitine and energy production -
The mitochondrial availability of carnitine during high-intensity exercise can be limited. After strenuous exercise, carnitine is believed to be a protective effect against muscle disruption.
It may help attenuate the release of creatine kinase and myoglobin. It may also help improve oxidative status and the protein signaling that is important for exercise recovery.
A review article by Gnoni and colleagues explored the potential role of L-carnitine supplementation in support of energy production during exercise. The authors describe a clinical trial where supplementation with 4 g of carnitine daily for 2 weeks in competitive walkers improved certain exercise-related parameters, including the maximum rate of oxygen consumption VO2max.
Other studies involving high performance athletes produced similar results. One study in junior athletes involved 1 g of carnitine supplementation for 6 weeks and reported higher athletic performances.
In untrained athletes , study participants experienced slight improvements in exercise performance in a study involving 2 g of carnitine daily for 2 weeks.
However, due to the relatively small sample size in these clinical trials, more research is needed to draw further conclusions. Carnitine plays other roles within the human body.
It has been shown to help support the transference of toxic compounds out of the mitochondria. It also may play a neuroprotective role. The acetyl derivative of L-carnitine, ALC, has been shown to pass through the blood-brain barrier at greater efficiency than L-carnitine.
ALC participates in glycogen synthesis, glucose metabolism modulation, increasing plasma adenosine triphosphate ATP concentration, and neurological function.
In particular, ALC facilitates cholinergic neurotransmission directly by providing an acetyl group for acetylcholine synthesis. Deschauer M, Wieser T, Zierz S: Muscle carnitine palmitoyltransferase II deficiency: clinical and molecular genetic features and diagnostic aspects.
Arch Neurol. Semba S, Yasujima H, Takano T, Yokozaki H: Autopsy case of the neonatal form of carnitine palmitoyltransferase-II deficiency triggered by a novel disease-causing mutation delC.
Pathol Int. Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D: Functional significance of cell volume regulatory mechanisms. Physiol Rev.
Berard E, Iordache A, Barrillon D, Bayle J: L-carnitine in dialysed patients: the choice of dosage regimen. Int J Clin Pharmacol Res. Wanner C, Forstner-Wanner S, Rossle C, Furst P, Schollmeyer P, Horl WH: Carnitine metabolism in patients with chronic renal failure: effect of L-carnitine supplementation.
Kidney Int Suppl. Wanner C, Horl WH: Carnitine abnormalities in patients with renal insufficiency. Pathophysiological and therapeutical aspects.
Calvani M, Benatti P, Mancinelli A, D'Iddio S, Giordano V, Koverech A, Amato A, Brass EP: Carnitine replacement in end-stage renal disease and hemodialysis. Handelman GJ: Debate forum: carnitine supplements have not been demonstrated as effective in patients on long-term dialysis therapy.
Blood Purif. Bertelli A, Giovannini L, Palla R, Migliori M, Panichi V, Andreini B: Protective effect of L-propionylcarnitine on cyclosporine-induced nephrotoxicity. Drugs Exp Clin Res. Origlia N, Migliori M, Panichi V, Filippi C, Bertelli A, Carpi A, Giovannini L: Protective effect of L-propionylcarnitine in chronic cyclosporine-a induced nephrotoxicity.
Biomed Pharmacother. Shores NJ, Keeffe EB: Is Oral L: -Acyl-Carnitine an Effective Therapy for Hepatic Encephalopathy? Review of the Literature. Dig Dis Sci.
Malaguarnera M, Gargante MP, Cristaldi E, Vacante M, Risino C, Cammalleri L, Pennisi G, Rampello L: Acetyl-L: -Carnitine Treatment in Minimal Hepatic Encephalopathy. Therrien G, Rose C, Butterworth J, Butterworth RF: Protective effect of L-carnitine in ammonia-precipitated encephalopathy in the portacaval shunted rat.
Casas H, Murtra B, Casas M, Ibanez J, Ventura JL, Ricart A, Rodriguez F, Viscor G, Palacios L, Pages T, Rama R: Increased blood ammonia in hypoxia during exercise in humans. J Physiol Biochem.
DaVanzo WJ, Ullian ME: L-carnitine administration reverses acute mental status changes in a chronic hemodialysis patient with hepatitis C infection.
Clin Nephrol. Malaguarnera M, Pistone G, Astuto M, Dell'Arte S, Finocchiaro G, Lo Giudice E, Pennisi G: L-Carnitine in the treatment of mild or moderate hepatic encephalopathy. Dig Dis. Malaguarnera M, Pistone G, Astuto M, Vecchio I, Raffaele R, Lo Giudice E, Rampello L: Effects of L-acetylcarnitine on cirrhotic patients with hepatic coma: randomized double-blind, placebo-controlled trial.
Malaguarnera M, Pistone G, Elvira R, Leotta C, Scarpello L, Liborio R: Effects of L-carnitine in patients with hepatic encephalopathy. World J Gastroenterol. Mullen KD, Gacad R: Pathogenetic mechanisms of acute hepatic encephalopathy. New Horiz. Pettegrew JW, Levine J, McClure RJ: Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer's disease and geriatric depression.
Mol Psychiatry. Rudman D, Sewell CW, Ansley JD: Deficiency of carnitine in cachectic cirrhotic patients. J Clin Invest. DeCarli LM, Lieber CS: Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet.
Klatskin G: Alcohol and its relation to liver damage. Sachan DS, Rhew TH, Ruark RA: Ameliorating effects of carnitine and its precursors on alcohol-induced fatty liver. Rhew TH, Sachan DS: Dose-dependent lipotropic effect of carnitine in chronic alcoholic rats.
Israel Y, Salazar I, Rosenmann E: Inhibitory effects of alcohol on intestinal amino acid transport in vivo and in vitro.
Kuhajda FP, Ronnett GV: Modulation of carnitine palmitoyltransferase-1 for the treatment of obesity. Curr Opin Investig Drugs. Aja S, Landree LE, Kleman AM, Medghalchi SM, Vadlamudi A, McFadden JM, Aplasca A, Hyun J, Plummer E, Daniels K, Kemm M, Townsend CA, Thupari JN, Kuhajda FP, Moran TH, Ronnett GV: Pharmacological stimulation of brain carnitine palmitoyl-transferase-1 decreases food intake and body weight.
Am J Physiol Regul Integr Comp Physiol. Obici S, Feng Z, Arduini A, Conti R, Rossetti L: Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production.
Nat Med. Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L: Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. He W, Lam TK, Obici S, Rossetti L: Molecular disruption of hypothalamic nutrient sensing induces obesity.
Nat Neurosci. Landree LE, Hanlon AL, Strong DW, Rumbaugh G, Miller IM, Thupari JN, Connolly EC, Huganir RL, Richardson C, Witters LA, Kuhajda FP, Ronnett GV: C75, a fatty acid synthase inhibitor, modulates AMP-activated protein kinase to alter neuronal energy metabolism.
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L: Central administration of oleic acid inhibits glucose production and food intake. Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, Long J, Sharman E, Liu J: Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats.
J Cell Mol Med. Goodman SI, Markey SP, Moe PG, Miles BS, Teng CC: Glutaric aciduria; a "new" disorder of amino acid metabolism. Biochem Med. Kolker S, Koeller DM, Okun JG, Hoffmann GF: Pathomechanisms of neurodegeneration in glutaryl-CoA dehydrogenase deficiency.
Ann Neurol. Baric I, Zschocke J, Christensen E, Duran M, Goodman SI, Leonard JV, Muller E, Morton DH, Superti-Furga A, Hoffmann GF: Diagnosis and management of glutaric aciduria type I.
Monavari AA, Naughten ER: Prevention of cerebral palsy in glutaric aciduria type 1 by dietary management. Arch Dis Child. Strauss KA, Puffenberger EG, Robinson DL, Morton DH: Type I glutaric aciduria, part 1: natural history of 77 patients. Am J Med Genet C Semin Med Genet.
Yannicelli S, Rohr F, Warman ML: Nutrition support for glutaric acidemia type I. J Am Diet Assoc. Maebashi M, Kawamura N, Sato M, Imamura A, Yoshinaga K: Urinary excretion of carnitine in patients with hyperthyroidism and hypothyroidism: augmentation by thyroid hormone.
Sima AA: Acetyl-L-carnitine in diabetic polyneuropathy: experimental and clinical data. CNS Drugs. discussion Tze WJ, Sima AA, Tai J: Effect of endocrine pancreas allotransplantation on diabetic nerve dysfunction.
Ward JD, Barnes CG, Fisher DJ, Jessop JD, Baker RW: Improvement in nerve conduction following treatment in newly diagnosed diabetics. Sima AA: C-peptide and diabetic neuropathy. Expert Opin Investig Drugs.
Sima AA, Bril V, Nathaniel V, McEwen TA, Brown MB, Lattimer SA, Greene DA: Regeneration and repair of myelinated fibers in sural-nerve biopsy specimens from patients with diabetic neuropathy treated with sorbinil.
N Engl J Med. Biolo G, Toigo G, Ciocchi B, Situlin R, Iscra F, Gullo A, Guarnieri G: Metabolic response to injury and sepsis: changes in protein metabolism. Famularo G, De Simone C, Trinchieri V, Mosca L: Carnitine and its congeners: a metabolic pathway to the regulation of immune response and inflammation.
Famularo G, De Simone C: A new era for carnitine?. Immunol Today. Penn D, Zhang L, Bobrowski PJ, Quinn M, Liu X, McDonough KH: Carnitine deprivation adversely affects cardiovascular response to bacterial endotoxin LPS in the anesthetized neonatal pig. Trumbeckaite S, Opalka JR, Neuhof C, Zierz S, Gellerich FN: Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function.
Eur J Biochem. Eaton S, Fukumoto K, Stefanutti G, Spitz L, Zammit VA, Pierro A: Myocardial carnitine palmitoyltransferase I as a target for oxidative modification in inflammation and sepsis.
Biochem Soc Trans. Nanni G, Pittiruti M, Giovannini I, Boldrini G, Ronconi P, Castagneto M: Plasma carnitine levels and urinary carnitine excretion during sepsis. JPEN J Parenter Enteral Nutr. Cederblad G, Larsson J, Nordstrom H, Schildt B: Urinary excretion of carnitne in burned patients.
McCarty MF, Rubin EJ: Rationales for micronutrient supplementation in diabetes. Med Hypotheses. Arslan E, Basterzi Y, Aksoy A, Majka C, Unal S, Sari A, Demirkan F: The additive effects of carnitine and ascorbic acid on distally burned dorsal skin flap in rats.
Med Sci Monit. Koybasi S, Taner Y: The Effect of L-Carnitine on Wound Healing by Secondary Intention in an Animal Model. Khan L, Bamji MS: Plasma carnitine levels in children with protein-calorie malnutrition before and after rehabilitation. Clin Chim Acta. Khan L, Bamji MS: Tissue carnitine deficiency due to dietary lysine dificiency: triglyceride accumulation and concomitant impairment in fatty acid oxidation.
Alp H, Orbak Z, Akcay F, Tan H, Aksoy H: Plasma and urine carnitine levels and carnitine supplementation in children with malnutrition. J Trop Pediatr. Wennberg A, Hyltander A, Sjoberg A, Arfvidsson B, Sandstrom R, Wickstrom I, Lundholm K: Prevalence of carnitine depletion in critically ill patients with undernutrition.
Winter SC, Szabo-Aczel S, Curry CJ, Hutchinson HT, Hogue R, Shug A: Plasma carnitine deficiency. Clinical observations in 51 pediatric patients. Am J Dis Child. Luci S, Geissler S, Konig B, Koch A, Stangl GI, Hirche F, Eder K: PPARalpha agonists up-regulate organic cation transporters in rat liver cells.
Koch A, Konig B, Luci S, Stangl GI, Eder K: Dietary oxidised fat up regulates the expression of organic cation transporters in liver and small intestine and alters carnitine concentrations in liver, muscle and plasma of rats. Br J Nutr. Karlic H, Schuster D, Varga F, Klindert G, Lapin A, Haslberger A, Handschur M: Vegetarian Diet Affects Genes of Oxidative Metabolism and Collagen Synthesis.
Traina G, Bernardi R, Cataldo E, Macchi M, Durante M, Brunelli M: In the Rat Brain Acetyl-L: -carnitine Treatment Modulates the Expression of Genes Involved in Neuronal Ceroid Lipofuscinosis. Mol Neurobiol. Mole SE, Williams RE, Goebel HH: Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses.
Pearce DA, Carr CJ, Das B, Sherman F: Phenotypic reversal of the btn1 defects in yeast by chloroquine: a yeast model for Batten disease. Pueschel SM: The effect of acetyl-L-carnitine administration on persons with Down syndrome.
Res Dev Disabil. Mancuso C, Bates TE, Butterfield DA, Calafato S, Cornelius C, De Lorenzo A, Dinkova Kostova AT, Calabrese V: Natural antioxidants in Alzheimer's disease. Ramassamy C: Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets.
Eur J Pharmacol. Palmieri F: Diseases caused by defects of mitochondrial carriers: a review. Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, Vazquez CM: The Role of Inflammatory Markers in the Cardioprotective Effect of L-Carnitine in L-NAME-Induced Hypertension.
Am J Hypertens. Diaz R, Lorita J, Soley M, Ramirez I: Carnitine worsens both injury and recovery of contractile function after transient ischemia in perfused rat heart.
Arsenian MA: Carnitine and its derivatives in cardiovascular disease. Prog Cardiovasc Dis. Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C: Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: a review.
Iliceto S, Scrutinio D, Bruzzi P, D'Ambrosio G, Boni L, Di Biase M, Biasco G, Hugenholtz PG, Rizzon P: Effects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: the L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico CEDIM Trial.
J Am Coll Cardiol. Colonna P, Iliceto S: Myocardial infarction and left ventricular remodeling: results of the CEDIM trial. Carnitine Ecocardiografia Digitalizzata Infarto Miocardico. Am Heart J. Tarantini G, Scrutinio D, Bruzzi P, Boni L, Rizzon P, Iliceto S: Metabolic treatment with L-carnitine in acute anterior ST segment elevation myocardial infarction.
A randomized controlled trial. Singh RB, Niaz MA, Agarwal P, Beegum R, Rastogi SS, Sachan DS: A randomised, double-blind, placebo-controlled trial of L-carnitine in suspected acute myocardial infarction.
Postgrad Med J. Xue YZ, Wang LX, Liu HZ, Qi XW, Wang XH, Ren HZ: L-carnitine as an adjunct therapy to percutaneous coronary intervention for non-ST elevation myocardial infarction.
Cardiovasc Drugs Ther. Nardin RA, Johns DR: Mitochondrial dysfunction and neuromuscular disease. Muscle Nerve. Borum PR, Broquist HP, Roelops RJ: Muscle carnitine levels in neuromuscular disease. J Neurol Sci. DiMauro S, DiMauro PM: Muscle carnitine palmityltransferase deficiency and myoglobinuria.
Lheureux PE, Penaloza A, Zahir S, Gris M: Science review: carnitine in the treatment of valproic acid-induced toxicity - what is the evidence?. Crit Care. Murphy JV, Marquardt KM, Shug AL: Valproic acid associated abnormalities of carnitine metabolism.
Spiller HA, Krenzelok EP, Klein-Schwartz W, Winter ML, Weber JA, Sollee DR, Bangh SA, Griffith JR: Multicenter case series of valproic acid ingestion: serum concentrations and toxicity.
J Toxicol Clin Toxicol. Watson WA, Litovitz TL, Klein-Schwartz W, Rodgers GC, Youniss J, Reid N, Rouse WG, Rembert RS, Borys D: annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med.
DeVivo DC: Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Konig SA, Siemes H, Blaker F, Boenigk E, Gross-Selbeck G, Hanefeld F, Haas N, Kohler B, Koelfen W, Korinthenberg R: Severe hepatotoxicity during valproate therapy: an update and report of eight new fatalities.
Siemes H, Nau H, Schultze K, Wittfoht W, Drews E, Penzien J, Seidel U: Valproate VPA metabolites in various clinical conditions of probable VPA-associated hepatotoxicity. Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Shabanah OA: Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model.
Pharmacol Res. Mandavilli BS, Santos JH, Van Houten B: Mitochondrial DNA repair and aging. Mutat Res. Costell M, O'Connor JE, Grisolia S: Age-dependent decrease of carnitine content in muscle of mice and humans. Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN: Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity.
Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN: Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress.
Sugiyama S: HMG CoA reductase inhibitor accelerates aging effect on diaphragm mitochondrial respiratory function in rats. Biochem Mol Biol Int. Sethumadhavan S, Chinnakannu P: L-carnitine and alpha-lipoic acid improve age-associated decline in mitochondrial respiratory chain activity of rat heart muscle.
J Gerontol A Biol Sci Med Sci. Kumaran S, Subathra M, Balu M, Panneerselvam C: Supplementation of L-carnitine improves mitochondrial enzymes in heart and skeletal muscle of aged rats. Exp Aging Res. Kumaran S, Panneerselvam KS, Shila S, Sivarajan K, Panneerselvam C: Age-associated deficit of mitochondrial oxidative phosphorylation in skeletal muscle: role of carnitine and lipoic acid.
Mol Cell Biochem. Colucci S, Mori G, Vaira S, Brunetti G, Greco G, Mancini L, Simone GM, Sardelli F, Koverech A, Zallone A, Grano M: L-carnitine and isovaleryl L-carnitine fumarate positively affect human osteoblast proliferation and differentiation in vitro.
Calcif Tissue Int. Maccari F, Arseni A, Chiodi P, Ramacci MT, Angelucci L: Levels of carnitines in brain and other tissues of rats of different ages: effect of acetyl-L-carnitine administration. Exp Gerontol. Adamek G, Felix R, Guenther HL, Fleisch H: Fatty acid oxidation in bone tissue and bone cells in culture.
Characterization and hormonal influences. Hooshmand S, Balakrishnan A, Clark RM, Owen KQ, Koo SI, Arjmandi BH: Dietary l-carnitine supplementation improves bone mineral density by suppressing bone turnover in aged ovariectomized rats. Corrales RM, Luo L, Chang EY, Pflugfelder SC: Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells.
De Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC: Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma.
Invest Ophthalmol Vis Sci. Goto E, Yagi Y, Matsumoto Y, Tsubota K: Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. Pflugfelder SC, Tseng SC, Sanabria O, Kell H, Garcia CG, Felix C, Feuer W, Reis BL: Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation.
Simmons P, Chang-Lin J, Chung Q, Vehige J, Welty D: Effect of compatible solutes on transepithelial electrical resistance and uptake in primary rabbit corneal epithelial cell layers model.
Association for Research in Vision and Ophthalmology ARVO Annual Meeting. Pescosolido N, Imperatrice B, Koverech A, Messano M: L-carnitine and short chain ester in tears from patients with dry eye.
Optom Vis Sci. Gilbard JP: Human tear film electrolyte concentrations in health and dry-eye disease. Int Ophthalmol Clin. Pessotto P, Liberati R, Petrella O, Romanelli L, Calvani M, Peluso G: In experimental diabetes the decrease in the eye of lens carnitine levels is an early important and selective event.
Exp Eye Res. Roomets E, Kivela T, Tyni T: Carnitine palmitoyltransferase I and Acyl-CoA dehydrogenase 9 in retina: insights of retinopathy in mitochondrial trifunctional protein defects. Tyni T, Kivela T, Lappi M, Summanen P, Nikoskelainen E, Pihko H: Ophthalmologic findings in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency caused by the GC mutation: a new type of hereditary metabolic chorioretinopathy.
Gillingham M, Van Calcar S, Ney D, Wolff J, Harding C: Dietary management of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency LCHADD.
A case report and survey. Tyni T, Pihko H, Kivela T: Ophthalmic pathology in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency caused by the GC mutation.
Curr Eye Res. Stanley CA: Carnitine deficiency disorders in children. Download references. Institute for Eye Research, Sydney, New South Wales, Australia. School of Optometry and Vision Science, University of New South Wales, Sydney, Australia.
You can also search for this author in PubMed Google Scholar. Correspondence to Judith L Flanagan. The authors' responsibilities were as follows--QG and JLF conceived and researched the review; JLF drafted the review; QG, MDW, JV and PAS provided critical discussion and revision of the article for intellectual content, and approved the final version of the manuscript.
This article is published under license to BioMed Central Ltd. Reprints and permissions. Flanagan, J. et al. Role of carnitine in disease. Nutr Metab Lond 7 , 30 Download citation.
Received : 17 December Accepted : 16 April Published : 16 April Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Abstract Carnitine is a conditionally essential nutrient that plays a vital role in energy production and fatty acid metabolism. Introduction Carnitine β-hydroxy-γ- N -trimethylaminobutyric acid is widely distributed in food from animals sources but there is limited availability in plants [ 1 ].
Carnitine biosynthesis and metabolism Carnitine, a branched non-essential amino acid, is synthesized from the essential amino acids lysine and methionine.
Figure 1. Carnitine biosynthesis and metabolism. Full size image. Figure 2. Conclusion Carnitine as a nutritional supplement has, since the s, been promoted as beneficial in a number of disorders of human carnitine deficiency of impaired fatty acid oxidation, suggesting that nutritional or pharmacologic supplements of carnitine might be beneficial in some disorders [ ].
References Kendler BS: Carnitine: an overview of its role in preventive medicine. Article CAS Google Scholar De Vivo DC, Tein I: Primary and secondary disorders of carnitine metabolism. Article CAS Google Scholar Rebouche CJ, Chenard CA: Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites.
CAS Google Scholar Cave MC, Hurt RT, Frazier TH, Matheson PJ, Garrison RN, McClain CJ, McClave SA: Obesity, inflammation, and the potential application of pharmaconutrition. Article Google Scholar Rebouche CJ: Carnitine function and requirements during the life cycle.
CAS Google Scholar Lombard KA, Olson AL, Nelson SE, Rebouche CJ: Carnitine status of lactoovovegetarians and strict vegetarian adults and children. CAS Google Scholar Vaz FM, Wanders RJ: Carnitine biosynthesis in mammals.
Article CAS Google Scholar Rebouche C: Carnitine: Modern Nutrition in Health and Disease. Google Scholar Angelini C, Trevisan C, Isaya G, Pegolo G, Vergani L: Clinical varieties of carnitine and carnitine palmitoyltransferase deficiency. Article CAS Google Scholar Bellinghieri G, Santoro D, Calvani M, Mallamace A, Savica V: Carnitine and hemodialysis.
Article CAS Google Scholar Rebouche CJ, Seim H: Carnitine metabolism and its regulation in microorganisms and mammals. Article CAS Google Scholar Ahmad S: L-carnitine in dialysis patients. Article CAS Google Scholar Lopaschuk GD: Current Concepts in Carnitine Research.
Article CAS Google Scholar Orngreen MC, Olsen DB, Vissing J: Exercise tolerance in carnitine palmitoyltransferase II deficiency with IV and oral glucose.
Article CAS Google Scholar Sahlin K, Sallstedt EK, Bishop D, Tonkonogi M: Turning down lipid oxidation during heavy exercise--what is the mechanism?. Google Scholar Peluso G, Barbarisi A, Savica V, Reda E, Nicolai R, Benatti P, Calvani M: Carnitine: an osmolyte that plays a metabolic role.
Article CAS Google Scholar Shennan DB, Grant A, Ramsay RR, Burns C, Zammit VA: Characteristics of L-carnitine transport by lactating rat mammary tissue.
Article CAS Google Scholar Burwinkel B, Kreuder J, Schweitzer S, Vorgerd M, Gempel K, Gerbitz KD, Kilimann MW: Carnitine transporter OCTN2 mutations in systemic primary carnitine deficiency: a novel ArgGln mutation and a recurrent Argter mutation associated with an unconventional splicing abnormality.
Article CAS Google Scholar Reuter SE, Faull RJ, Evans AM: L-carnitine supplementation in the dialysis population: are Australian patients missing out?.
CAS Google Scholar Cederbaum SD, Koo-McCoy S, Tein I, Hsu BY, Ganguly A, Vilain E, Dipple K, Cvitanovic-Sojat L, Stanley C: Carnitine membrane transporter deficiency: a long-term follow up and OCTN2 mutation in the first documented case of primary carnitine deficiency.
Article CAS Google Scholar Wang Y, Kelly MA, Cowan TM, Longo N: A missense mutation in the OCTN2 gene associated with residual carnitine transport activity.
Article CAS Google Scholar Pons R, De Vivo DC: Primary and secondary carnitine deficiency syndromes. Google Scholar Koizumi A, Nozaki J, Ohura T, Kayo T, Wada Y, Nezu J, Ohashi R, Tamai I, Shoji Y, Takada G, Kibira S, Matsuishi T, Tsuji A: Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency.
Article CAS Google Scholar Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, Nikaido H, Sai Y, Koizumi A, Shoji Y, Takada G, Matsuishi T, Yoshino M, Kato H, Ohura T, Tsujimoto G, Hayakawa J, Shimane M, Tsuji A: Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter.
Article CAS Google Scholar Erguven M, Yilmaz O, Koc S, Caki S, Ayhan Y, Donmez M, Dolunay G: A case of early diagnosed carnitine deficiency presenting with respiratory symptoms.
Article CAS Google Scholar Mayatepek E, Nezu J, Tamai I, Oku A, Katsura M, Shimane M, Tsuji A: Two novel missense mutations of the OCTN2 gene WR and VF in a patient with primary systemic carnitine deficiency.
Article CAS Google Scholar Sigauke E, Rakheja D, Kitson K, Bennett MJ: Carnitine palmitoyltransferase II deficiency: a clinical, biochemical, and molecular review. Article CAS Google Scholar Vermeire S, Rutgeerts P: Current status of genetics research in inflammatory bowel disease.
Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis.
Eur Heart J. Rebouche CJ, Engel AG. Kinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes. Evidence for alterations in tissue carnitine transport. J Clin Invest. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, et al.
Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Muller D.
Plasma concentrations of Trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost G, et al.
A metabolomic study of biomarkers of meat and fish intake. Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Gillett MB, Suko JR, Santoso FO, Yancey PH. Elevated levels of trimethylamine oxide in muscles of deep-sea gadiform teleosts: a high-pressure adaptation?
J Exp Zool. Yancey PH, Gerringer ME, Drazen JC, Rowden AA, Jamieson A. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc Natl Acad Sci U S A. Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: a pilot study.
Food Chem Toxicol. Tong TYN, Appleby PN, Bradbury KE, Perez-Cornago A, Travis RC, Clarke R, Key TJ. Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: results from the prospective EPIC-Oxford study.
Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant.
Hauet T, Baumert H, Gibelin H, Godart C, Carretier M, Eugene M. Citrate, acetate and renal medullary osmolyte excretion in urine as predictor of renal changes after cold ischaemia and transplantation.
Clin Chem Lab Med. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL.
Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Bielinska K, Radkowski M, Grochowska M, Perlejewski K, Huc T, Jaworska K, Motooka D, Nakamura S, Ufnal M.
High salt intake increases plasma trimethylamine N-oxide TMAO concentration and produces gut dysbiosis in rats.
Jaworska K, Huc T, Samborowska E, Dobrowolski L, Bielinska K, Gawlak M, Ufnal M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite.
PLoS One. Xu M, Bhatt DK, Yeung CK, Claw KG, Chaudhry AS, Gaedigk A, Pearce RE, Broeckel U, Gaedigk R, Nickerson DA, et al.
Genetic and nongenetic factors associated with protein abundance of Flavin-containing Monooxygenase 3 in human liver. J Pharmacol Exp Ther. Ufnal M, Pham K. The gut-blood barrier permeability - a new marker in cardiovascular and metabolic diseases?
Med Hypotheses. Lango R, Smolenski RT, Narkiewicz M, Suchorzewska J, Lysiak-Szydlowska W. Influence of L-carnitine and its derivatives on myocardial metabolism and function in ischemic heart disease and during cardiopulmonary bypass.
Cardiovasc Res. Iliceto S, Scrutinio D, Bruzzi P, D'Ambrosio G, Boni L, Di Biase M, Biasco G, Hugenholtz PG, Rizzon P. Effects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: the L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico CEDIM trial.
J Am Coll Cardiol. Hiramatsu A, Aikata H, Uchikawa S, Ohya K, Kodama K, Nishida Y, Daijo K, Osawa M, Teraoka Y, Honda F, et al. Levocarnitine use is associated with improvement in sarcopenia in patients with liver cirrhosis.
Hepatol Commun. Hathcock JN, Shao A. Risk assessment for carnitine. Regul Toxicol Pharmacol. Shang R, Sun Z, Li H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta-analysis.
BMC Cardiovasc Disord. Bakalov D, Sabit Z, Tafradjiiska-Hadjiolova R. Re: effect of l-carnitine supplementation on muscle cramps induced by stroke: a case report. Download references. Department of Human Physiology, Faculty of Health Sciences, Medical University of Gdansk, , Gdansk, Poland.
You can also search for this author in PubMed Google Scholar. Conceptualization: R. and R. All authors have read and agreed to the published version of the manuscript. Correspondence to Robert A. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Sawicka, A. The bright and the dark sides of L-carnitine supplementation: a systematic review. J Int Soc Sports Nutr 17 , 49 Download citation. Received : 13 March Accepted : 04 September Published : 21 September Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Review Open access Published: 21 September The bright and the dark sides of L-carnitine supplementation: a systematic review Angelika K.
Olek ORCID: orcid. Abstract Background L-carnitine LC is used as a supplement by recreationally-active, competitive and highly trained athletes.
Methods A literature search was conducted in the MEDLINE via PubMed and Web of Science databases from the inception up February Results The initial search retrieved articles, and a total of 11 studies were finally included after applying inclusion and exclusion criteria.
Conclusion Prolonged LC supplementation in specific conditions may affect physical performance. Background The main function of L-carnitine LC is the transport of long-chain fatty acids into the mitochondrial matrix for their conversion in energy, via β-oxidation process [ 1 ]. Information sources and search The literature was explored using the MEDLINE via PubMed and Web of Science databases, including all articles published from the inception up February Study selection Firstly, studies were assessed by title verification between databases duplicates were removed.
Data collection process The following information was compiled for each study: authors, year of publication, type of study, length of supplementation, a dose of supplementation and main effect. Results Study selection By the above-described search strategy, publications were identified.
Flowchart on the search and selection of articles included in the review. Full size image. Table 1 Summary and results of the studies reviewed examining the LC supplementation Full size table.
Discussion The present findings have been debated in the six separate paragraphs, and for a better picture of LC supplementation, other studies were also disputed. Skeletal muscle protein balance regulation Skeletal muscle mass depends on the rates of protein synthesis and degradation.
Strengths and limitations The strength of this review is a focus on the period of LC treatment, very important aspect often missed in many articles dealing with this supplement. Conclusions Lasting for several years opinion that LC supplementation does not change metabolism, especially exercise metabolism, is based mostly on short-term supplementation protocols.
Availability of data and materials Not applicable. Abbreviations LC: L-carnitine TC: Total carnitine TMAO: Trimethylamine-N-oxide CHO: Carbohydrates IGF Insulin-like growth factor-1 PI3K: Phosphoinositidekinase Akt: Protein kinase B mTOR: Mammalian target of rapamycin S6K: S6 kinase 4E-BP: 4E-binding protein FoxO: Forkhead box O MuRF Muscle-specific RING finger-1 atrogin Muscle atrophy F-box mRNA: Messenger RNA BMI: Body mass index ROS: Reactive oxygen species.
References Bremer J. Article CAS PubMed Google Scholar Arenas J, Huertas R, Campos Y, Diaz AE, Villalon JM, Vilas E. Article CAS PubMed Google Scholar Ringseis R, Keller J, Eder K. Article CAS PubMed Google Scholar Brass EP. Article CAS PubMed Google Scholar Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL.
Article CAS PubMed PubMed Central Google Scholar Stephens FB, Wall BT, Marimuthu K, Shannon CE, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Article CAS PubMed PubMed Central Google Scholar Shannon CE, Ghasemi R, Greenhaff PL, Stephens FB. Article PubMed Google Scholar Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al.
Article CAS PubMed PubMed Central Google Scholar Baltazar-Martins G, Brito de Souza D, Aguilar-Navarro M, Munoz-Guerra J, MDM P, Del Coso J. Article CAS PubMed PubMed Central Google Scholar Wardenaar FC, Ceelen IJ, Van Dijk JW, Hangelbroek RW, Van Roy L, Van der Pouw B, De Vries JH, Mensink M, Witkamp RF.
Article CAS PubMed Google Scholar Wachter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, Krahenbuhl S. Article CAS PubMed Google Scholar Novakova K, Kummer O, Bouitbir J, Stoffel SD, Hoerler-Koerner U, Bodmer M, Roberts P, Urwyler A, Ehrsam R, Krahenbuhl S.
Article CAS PubMed Google Scholar Lohninger A, Sendic A, Litzlbauer E, Hofbauer R, Staniek H, Blesky D, Schwieglhofer C, Eder M, Bergmuller H, Mascher D, et al. Article CAS Google Scholar Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M. Article CAS PubMed Google Scholar Sawicka AK, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek RA.
Article CAS PubMed Google Scholar Olek RA, Samulak JJ, Sawicka AK, Hartmane D, Grinberga S, Pugovics O, Lysiak-Szydlowska W. Article CAS Google Scholar Bordoni L, Sawicka AK, Szarmach A, Winklewski PJ, Olek RA, Gabbianelli R. Article CAS Google Scholar Grunewald KK, Bailey RS.
Article CAS PubMed Google Scholar Hawley JA, Brouns F, Jeukendrup A. Article CAS PubMed Google Scholar Barnett C, Costill DL, Vukovich MD, Cole KJ, Goodpaster BH, Trappe SW, Fink WJ.
Article CAS PubMed Google Scholar Vukovich MD, Costill DL, Fink WJ. Article CAS Google Scholar Rebouche CJ. Article CAS PubMed Google Scholar Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL.
Article CAS PubMed Google Scholar Stephens FB, Evans CE, Constantin-Teodosiu D, Greenhaff PL. Article CAS Google Scholar Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. Article CAS PubMed Google Scholar Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M.
Article CAS PubMed Google Scholar Sanchez AM, Candau RB, Bernardi H. Article CAS PubMed Google Scholar Keller J, Ringseis R, Priebe S, Guthke R, Kluge H, Eder K. Article CAS PubMed Google Scholar Keller J, Ringseis R, Koc A, Lukas I, Kluge H, Eder K.
Article CAS PubMed Google Scholar Busquets S, Serpe R, Toledo M, Betancourt A, Marmonti E, Orpi M, Pin F, Capdevila E, Madeddu C, Lopez-Soriano FJ, et al. Article CAS PubMed Google Scholar Keller J, Couturier A, Haferkamp M, Most E, Eder K.
Article CAS Google Scholar Keller J, Ringseis R, Eder K. Article CAS PubMed PubMed Central Google Scholar Jang J, Park J, Chang H, Lim K. Article CAS PubMed Google Scholar Di Marzio L, Moretti S, D'Alo S, Zazzeroni F, Marcellini S, Smacchia C, Alesse E, Cifone MG, De Simone C.
Article CAS PubMed Google Scholar Kraemer WJ, Volek JS, French DN, Rubin MR, Sharman MJ, Gomez AL, Ratamess NA, Newton RU, Jemiolo B, Craig BW, et al. Article PubMed Google Scholar Rondanelli M, Solerte SB, Fioravanti M, Scevola D, Locatelli M, Minoli L, Ferrari E.
Article CAS PubMed Google Scholar Evans M, Guthrie N, Pezzullo J, Sanli T, Fielding RA, Bellamine A. Article CAS Google Scholar Askarpour M, Hadi A, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E.
Article CAS PubMed Google Scholar Lee JK, Lee JS, Park H, Cha YS, Yoon CS, Kim CK. Article CAS PubMed Google Scholar Rafraf M, Karimi M, Jafari A. CAS PubMed Google Scholar Koozehchian MS, Daneshfar A, Fallah E, Agha-Alinejad H, Samadi M, Kaviani M, Kaveh BM, Jung YP, Sablouei MH, Moradi N, et al.
Article PubMed PubMed Central Google Scholar Ahlborg G, Jensen-Urstad M. Article CAS PubMed Google Scholar Doherty TJ. Article CAS Google Scholar Volpato S, Bianchi L, Cherubini A, Landi F, Maggio M, Savino E, Bandinelli S, Ceda GP, Guralnik JM, Zuliani G, et al. Article CAS PubMed Google Scholar Peake J, Suzuki K.
PubMed Google Scholar Peake J, Nosaka K, Suzuki K. PubMed Google Scholar Fritz IB, Arrigoni-Martelli E. Article CAS PubMed Google Scholar Giamberardino MA, Dragani L, Valente R, Di Lisa F, Saggini R, Vecchiet L. Article CAS PubMed Google Scholar Volek JS, Kraemer WJ, Rubin MR, Gomez AL, Ratamess NA, Gaynor P.
Article CAS PubMed Google Scholar Spiering BA, Kraemer WJ, Vingren JL, Hatfield DL, Fragala MS, Ho JY, Maresh CM, Anderson JM, Volek JS. Article PubMed Google Scholar Ho JY, Kraemer WJ, Volek JS, Fragala MS, Thomas GA, Dunn-Lewis C, Coday M, Hakkinen K, Maresh CM. Article CAS PubMed Google Scholar Spiering BA, Kraemer WJ, Hatfield DL, Vingren JL, Fragala MS, Ho JY, Thomas GA, Hakkinen K, Volek JS.
Article PubMed Google Scholar Rebouche CJ, Mack DL, Edmonson PF. Article CAS Google Scholar Rebouche CJ, Chenard CA. Article CAS PubMed Google Scholar Fukami K, Yamagishi S, Sakai K, Kaida Y, Yokoro M, Ueda S, Wada Y, Takeuchi M, Shimizu M, Yamazaki H, et al.
Article CAS PubMed Google Scholar Vallance HD, Koochin A, Branov J, Rosen-Heath A, Bosdet T, Wang Z, Hazen SL, Horvath G. Article CAS PubMed PubMed Central Google Scholar Samulak JJ, Sawicka AK, Samborowska E, Olek RA.
Article CAS PubMed PubMed Central Google Scholar Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Article PubMed PubMed Central Google Scholar Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL.
Article CAS PubMed PubMed Central Google Scholar Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Article CAS PubMed Google Scholar Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Article CAS PubMed Google Scholar Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJL, Dullaart RPF.
Article CAS PubMed PubMed Central Google Scholar Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Article CAS PubMed Google Scholar Rebouche CJ, Engel AG. Article CAS PubMed PubMed Central Google Scholar Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, et al.
Carnitine is a quaternary Antibacterial body wash L-carnitine and energy production involved L-carnitine and energy production metabolism in most mammals, L-carhitine, and some bacteria. Some individuals with genetic or medical produchion L-carnitine and energy production as preterm infants cannot enerfy enough carnitine, requiring dietary supplementation. Many eukaryotes Mood enhancing lifestyle the ability to synthesize carnitine, including prdouction. HTML is then cleaved by HTML aldolase HTMLA, a pyridoxal phosphate requiring enzymeyielding 4-trimethylaminobutyraldehyde TMABA and glycine. Carnitine is involved in transporting fatty acids across the mitochondrial membrane, by forming a long chain acetylcarnitine ester and being transported by carnitine palmitoyltransferase I and carnitine palmitoyltransferase II. The tissue distribution of carnitine-biosynthetic enzymes in humans indicates TMLD to be active in the liver, heart, muscle, brain and highest in the kidneys. The rate of TMABA oxidation is greatest in the liver, with considerable activity also in the kidneys. Stress reduction properties, also L-carnitine and energy production L-carnitin levocarnitine, is a naturally occurring amino acid structure that Skin rejuvenation for uneven skin tone body produces. L-carnitine pgoduction a critical role in energy production, as it L-carnittine fat into energy. Those with low L-carnitine levels may benefit from taking an oral supplement, though. As well as supporting energy production, L-carnitine may help some other functions in the body, such as maintaining general brain function and reducing the risk of certain disorders. Some people may experience mild side effects when increasing their L-carnitine intake, especially with long-term use.
Video
EHPlabs Acetyl L-Carnitine - Supports Natural Energy Production, Aids MetabolismL-carnitine and energy production -
In conclusion, ploidy significantly affected the energy metabolism in rainbow trout, dietary l -carnitine levels altered the l -carnitine homeostasis, but not influence nutritional metabolism.
Abdallah Tageldein Mansour, Ahmed H. Arisha, … Walaa El-Houseiny. Hirofumi Furuita, Tadao Jinbo, … Hideki Tanaka. Fish meal and fish oil derived from wild catches are dominant ingredients in aqua feed.
It is estimated, at the present annual growth rates, that fishmeal supplies will be entirely consumed by the aquaculture sector by Plant protein ingredients have increasingly been used in diets of aquaculture species Glencross Booth and Allan , including carnivore marine species Oliva-Teles and Gonçalves ; Colburn et al.
The replacement of fish meal may trigger, however, specific deficiency symptoms, including l -carnitine deficiency. Fishmeal may contain 10—20 times more l -carnitine than plant-based feeds Ozório et al. l -Carnitine, a multi-physiological and bioactive vitamin-like nutrient, plays an important role in cellular nutritional metabolic status, including the regulation of fish energy metabolism and growth Harpaz ; Mohseni et al.
Fish fed high-fat diets supplemented with mg l -carnitine showed a reduction in plasma glucose and lactate dehydrogenase LDH Ozório et al.
A decrease in LDH levels may also indicate an increase protection of l -carnitine against oxidative stress Ronca et al. According to Rasmussen and Wolfe growth is impaired when a dietary l -carnitine level is limited. In fish, an increase in energy demand is followed by the increase in lipid metabolism and body l -carnitine turnover Ozório et al.
Carnitine reserves are mainly replenished by the diet since carnitine biosynthesis is not sufficient, especially in very young animals Harpaz Ozório et al. juveniles fed l -carnitine supplements following acute exposure to toxic ammonia levels and to pathogen agent S. In case of fishmeal replacement, body l -carnitine reserves may become limited, thus dietary supplementation is required.
In salmonid production, sterile triploids have been increasingly used. After the onset of sexual maturation, triploid fish often grow faster and have better flesh quality than their diploid counterparts.
In addition, triploid fish may display greater hypertrophic growth and reduced energy costs for gamete production Thorgaard ; Qin et al. The comparative results between diploid and triploid fish are often contradictory Ihssen et al. The general consensus is that the survival and growth are substantially lower in the early life stages in triploids compared to diploids Thorgaard ; Sutterlin et al.
In addition, triploids appear to be less resilient and less tolerant to poor water quality than their diploid counterpart Benfey ; Benfey and Biron ; Altimiras et al. The aim of this study was to assess the effects of dietary l -carnitine levels on energy metabolism in diploid and triploid rainbow trout Oncorhynchus mykiss.
The current study is an extension of a published study Ozório et al. Two groups of 5-month-old rainbow trout were used, one of all-female diploid trout initial weight After an acclimatization period of 2 weeks, rainbow trout were individually weighed and randomly distributed in 18 fiberglass tanks of 50 L.
The water supply system consisted of a closed circuit TMC, TMC Iberia, Lisbon, Portugal , which included mechanical and biological filtration, a skimmer, an ultraviolet sterilization unit and a refrigerator.
The water quality parameters such as temperature Fish were fed twice daily and h , to apparent satiation. At the end of the trial all fish were starved for 24 h prior to sampling.
Before sampling, fish were euthanized by an overdose bath of 0. A total of 12 fish per group were sampled for muscle, liver and blood plasma analyses. The results of growth performance parameters are presented solely in the text of the Result section.
For detailed information about the growth performance, see Ozório et al. The voluntary feed intake VFI , feed conversion ratio FCR and growth rate daily growth index, DGI was calculated as follows:.
Diets, liver, and muscle tissues were analyzed for the free- and acyl l -carnitine content. All samples, prior to l -carnitine analysis, were deproteinated with perchloric acid, subsequently neutralized with potassium hydroxide, centrifuged and the supernatant collected.
l -Carnitine was extracted by stepwise heating, ultrasonic treatment or extraction with various detergents. Additionally, alkaline hydrolysis was performed for the determination of l -carnitine esters.
The l -carnitine determination in diets and tissues was performed by radiometric detection of free and l -carnitine esters. The assay is based on the reaction of free l -carnitine with acetyl-CoA catalyzed by carnitine acetyltransferase with the production of acetyl carnitine and coenzyme Christiansen and Bremer Blood was collected by caudal puncture with lithium-heparin coated syringes.
All plasma tests were performed in triplicate. The total ammonia concentration in plasma was determined using the method described by Bergmeyer and Beutler and plasma osmotic pressure mOsm was determined with an osmometer model 15—Löser Messtechnik, Berlin, Germany.
Plasma glucose concentrations were measured by the glucose oxidase method using a Beckman glucose analyzer 2 Beckman Instruments, Brea, CA, USA. Urea was quantified colourimetrically with the Glutamate dehydrogenase GLDH enzymatic method. Alanine aminotransferase ALT was quantified by an enzymatic method in accordance with IFCC.
Lactate dehydrogenase LDH was quantified by an enzymatic method in accordance with IFCC. All the methods were executed in a Roche Cobas Integra Chemistry Analyser Roche ® Sistemas de Diagnóstico, Amadora, Portugal , with Roche reagents.
Triiodothyronine T3 and thyroxine T4 were quantified by electrochemiluminescence immunoassays in a Roche Cobas e Analyser Roche ® Sistemas de Diagnóstico, Amadora, Portugal , with Roche reagents. All data were tested for normality using Kolmogorov—Smirnov and Levene tests and analyzed using two-way analyses of variance ANOVA.
The model included the effect of ploidy two levels and dietary l -carnitine three levels and their interaction. Data were analyzed using the General Linear Model procedure SAS Institute Inc.
Voluntary feed intake VFI: 1. Feed conversion ratio FCR: 0. Growth rate 2. The concentration of l -carnitine fractions free and acyl l -carnitine significantly increased in muscle and liver Fig. Free carnitine content was significantly higher than acyl l -carnitine content in both muscle and liver tissues.
Acyl l -carnitine, free l -carnitine and total carnitine content in muscle a and liver b of diploid and triploid rainbow trout fed the experimental diets. Ploidy and l -carnitine supplementation did not affect plasma glucose, urea, protein, and triglyceride levels Table 2. Trout fed mg l -carnitine had the lowest ammonia 8.
Plasma cortisol ranged from 4. In studies with larger animals, the positive effects of l -carnitine become less evident, as confirmed by Chatzifotis et al.
In the current study using juveniles, with exception of FCR, l -carnitine supplementation did not improve growth performance.
The ambiguity among many dose—response studies on l -carnitine may be caused by different l -carnitine concentrations, the husbandry conditions or the duration of the dietary treatment Ozório et al. The carnitine pool in animals is maintained by a combination of absorption of carnitine from supplemental sources, a modest rate of biosynthesis, and an efficient reabsorption of carnitine Rebouche and Seim The body distribution of carnitine is determined by a series of systems that transport carnitine into cells against a concentration gradient.
The liver plays a unique role in the whole-body carnitine homeostasis. Free and acyl l -carnitine content increased with the dietary l -carnitine supplementation, whereas higher levels were observed in muscle than in liver, indicating a direct relationship between the dietary and body l -carnitine.
The muscle may store a large amount of l -carnitine against a concentration gradient by an active transport process, which could be to ensure an immediate and efficient energy for the muscle during exercise and recovery.
The l -carnitine concentration gradient was even more pronounced between muscle and liver tissues Ozório et al. The ability to take up and retain dietary carnitine was observed in other species, such as Atlantic salmon Salmo salar L.
Ji et al. The increase in the acyl l -carnitine fraction in fish tissues may indicate an increase in lipid catabolism and that the diet quality and quantity may affect the body l -carnitine level. These results are consistent with the study described by Ozório et al. In fact, muscle l -carnitine levels increased moderately when African catfish were fed between 40 and mg l -carnitine.
Thereafter, a sharp increase in muscle l -carnitine level was observed. In the current study, the ploidy affected the acyl l -carnitine content in muscle, with higher values observed in triploid fish. This could indicate that triploid trout have higher l -carnitine requirement than diploid fish.
In fact, triploid fish have a lower respiratory efficiency and are more susceptible to stress than diploid fish. Triploid fish have fewer red blood cells which probably affect the exchange of oxygen and decrease the ability to transport oxygen in the blood Benfey ; Benfey and Biron ; Tiwary and Ray and, therefore, spend more energy to maintain internal balance than their diploid counterpart, possibly mobilizing more body l -carnitine for energy production.
Plasma lipase was higher in diploid fish and is in agreement with the slight increase in plasma triglyceride. Lipase is the rate-limiting enzyme for the hydrolysis of triglyceride in plasma Albalat et al.
In the current study, triploid trout had lower plasma ammonia concentrations, which may reflect on a higher capacity to excrete ammonia than diploid trout.
Considering that ammonia excretion rates are linked to acid—base regulation Kieffer and Tufts , triploid rainbow trout may have a greater clearance capacity of metabolic protons. This would allow for faster recovery from acidosis and energy ATP depletion after exercise, which is often the case during handling stress.
A faster recovery of the blood acid—base status may also reflect a faster recovery of ion and osmoregulatory imbalance, which was also shown by the significant differences in plasma osmolality between triploid and diploid trout. Plasma triiodothyronine hormone T 3 was significantly higher in diploid than triploid trout, as well as the feed intake VFI.
Feed intake, quality and quantity, is known to influence thyroid hormone levels in rainbow trout Leatherland et al. Thyroid hormones interact closely with the energy metabolism, with T 3 playing a role in the regulation of growth and nutrient partitioning in teleosts Peter and Marchant ; Korytko and Cuttler In the current study triploid trout had lower plasma LDH level.
As mentioned earlier, triploid fish have lower oxygen transport ability, which may explain their lower activity when compared to diploid trout. According to Sullivan and Somero , fish with different locomotors activity would have different LDH activity to compensate for the amount of lactate produced.
Wardle reported lower lactate ion concentration in flatfish than in rainbow trout. Although a multitude of questions concerning the regulation of dietary carnitine requirements remain to be elucidated, our study suggested that ploidy significantly affected the energy metabolism, whereas dietary l -carnitine levels altered the carnitine homeostasis, but did not influence energy metabolism in diploid and triploid rainbow trout.
Albalat A, Sánchez-Gurmaches J, Gutiérrez J, Navarro I Regulation of lipoprotein lipase activity in rainbow trout Oncorhynchus mykiss tissues. Gen Comp Endocrinol — Article CAS Google Scholar.
Altimiras J, Axelsson M, Claireaux G, Lefrançois C, Mercier C, Farrell AP Cardiorespiratory status of triploid brown trout during swimming at two acclimation temperatures. J Fish Biol — Article Google Scholar.
Atkins ME, Benfey TJ Effect of acclimation temperature on routine metabolic rate in triploid salmonids. Comp Biochem Physiol A Mol Integr Physiol — Bell FP, Vidmar TJ, Raymond TL l -Carnitine administration and withdrawal affect plasma and hepatic carnitine concentrations, plasma lipid and lipoprotein composition, and in vitro hepatic lipogenesis from labeled mevalonate and oleate in normal rabbits.
J Nutr — Benfey TJ The physiology and behavior of triploid fishes. Rev Fish Sci — Benfey T, Biron M Acute stress response in triploid rainbow trout Oncorhynchus mykiss and brook trout Salvelinus fontinalis. Aquaculture — Bergmeyer HU, Beutler HO Ammonia.
Methods Enzymat Anal — Google Scholar. Böhles H, Michalk D, Brandl U, Fekl W, Börresen HC, Stehr K The effect of l -carnitine-supplemented total parenteral nutrition on tissue amino acid concentrations in piglets.
Chatzifotis S, Takeuchi T Effect of supplemental carnitine on body weight loss, proximate and lipid compositions and carnitine content of red sea bream Pagrus major during starvation. Chatzifotis S, Takeuchi T, Seikai T The effect of dietary l -carnitine on growth performance and lipid composition in red sea bream fingerlings.
Fish Sci — Chatzifotis S, Takeuchi T, Seikai T The effect of dietary carnitine supplementation on growth of red sea bream Pagrus major fingerlings at two levels of dietary lysine.
Christiansen RZ, Bremer J Acetylation of tris hydroxymethyl aminomethane tris and tris derivatives by carnitine acetyltransferase. FEBS Lett — Colburn HR, Walker AB, Breton TS, Stilwell JM, Sidor IF, Gannam AL, Berlinsky DL Partial replacement of fishmeal with soybean meal and soy protein concentrate in diets of atlantic cod.
N Am J Aquacult — FAO The state of world fisheries and aquaculture. FAO, Rome. Galbreath PF, St Jean W, Anderson V, Thorgaard GH Freshwater performance of all-female diploid and triploid atlantic salmon. Glencross BD, Booth M, Allan GL A feed is only as good as its ingredients—a review of ingredient evaluation strategies for aquaculture feeds.
Aquacult Nutr — Harpaz S l -Carnitine and its attributed functions in fish culture and nutrition—a review. Himick BA, Higgs DA, Eales JG The acute effects of alteration in the dietary concentrations of carbohydrate, protein, and lipid on plasma t4, t3, and glucose levels in rainbow trout, Oncorhynchus mykiss.
Hokland BM Uptake, metabolism and release of carnitine and acylcarnitines in the perfused rat liver. Biochim Biophys Acta — Ihssen PE, McKay LR, McMillan I, Phillips RB Ploidy manipulation and gynogenesis in fishes: cytogenetic and fisheries applications. Trans Am Fish Soc — Ji H, Bradley TM, Tremblay GC Atlantic salmon Salmo salar fed l -carnitine exhibit altered intermediary metabolism and reduced tissue lipid, but no change in growth rate.
CAS Google Scholar. Kerner J, Hoppel C Genetic disorders of carnitine metabolism and their nutritional management. Annu Rev Nutr — Keshavanath P, Renuka P Effect of dietary l -carnitine on growth and body composition of fingerling rohu, Labeo rohita Hamilton. Kieffer JD, Tufts BL The influence of environmental temperature on the role of the rainbow trout gill in correcting the acid—base disturbance following exhaustive exercise.
Physiol Zool — Korytko AI, Cuttler L Thyroid hormone and glucocorticoid regulation of pituitary growth hormone-releasing hormone receptor gene expression. J Endocrinol R13—R Leatherland FJ, Cho CY, Slinger SJ Effects of diet, ambient temperature, and holding conditions on plasma thyroxine levels in rainbow trout Salmo gairdneri.
J Fish Res Board Can — Li JM, Li LY, Qin X, Ning LJ, Lu DL, Li DL, Zhang ML, Wang X, Du ZY Systematic regulation of l -carnitine in nutritional metabolism in zebra-fish, Danio rerio. Sci Rep Malison JA, Procarione LS, Held JA, Kayes TB, Amundson CH The influence of triploidy and heat and hydrostatic pressure shocks on the growth and reproductive development of juvenile yellow perch Perca jlavescens.
Elevated protein synthesis and attenuated proteolysis are observed during muscle hypertrophy. Both of these processes are mainly regulated by the signaling pathway: insulin-like growth factor-1 IGF-1 — phosphoinositidekinase PI3K — protein kinase B Akt — mammalian target of rapamycin mTOR.
The activation of mTOR leads to phosphorylation and activation of S6 kinases S6Ks and hyperphosphorylation of 4E-binding proteins 4E-BPs , resulting in the acceleration of protein synthesis. At the same time, Akt phosphorylates and inactivates forkhead box O FoxO , thereby inhibit the responsible for proteolysis ubiquitin ligases: muscle-specific RING finger-1 MuRF-1 and muscle atrophy F-box protein atrogin-1 , for review see [ 27 , 28 , 29 ].
The association between LC supplementation and the regulation of metabolic pathways involved in muscle protein balance have been shown in several animal studies Fig. Four weeks of LC supplementation in rats increased plasma IGF-1 concentration [ 33 ]. FoxO inactivation attenuated MURF-1 expression in quadriceps fem oris muscle of supplemented rats compared to control [ 33 ].
All these findings together might suggest that LC supplementation protect muscle from atrophy, especially in pathophysiological conditions. The association between LC supplementation and the regulation of metabolic pathways involved in muscle protein balance.
L-carnitine LC ; insulin-like growth factor-1 IGF-1 ; phosphoinositidekinase PI3K ; protein kinase B Akt ; mammalian target of rapamycin mTOR ; forkhead box O FoxO ; muscle-specific RING finger-1 MuRF-1 ; muscle atrophy F-box atrogin-1 ; increase ; decrease ; activation ; inactivation.
Various effects might be due to different IGF-1 levels; significantly lower in the HIV-seropositive patients than in healthy subjects [ 38 ]. These findings altogether suggest that prolonged LC supplementation might affect body composition in specific conditions.
Therefore, authors suggested that LC supplementation may be effective in obese and overweight subjects. It has been assumed that a combination of LC supplementation with increased energy expenditure may positively affect body composition.
However, either with aerobic [ 41 , 42 ] or resistance [ 43 ] training, LC supplementation has not achieved successful endpoint. Similarly, lack of LC effect has been reported in obese women [ 42 ]. Body composition, determined by dual energy X-ray absorptiometry, indicated no significant effect in fat mass and fat-free mass due to supplementation.
Moreover, LC administration did not influence bench press results. The number of leg press repetitions and the leg press third set lifting volume increased in the LC group compared to the placebo group [ 43 ].
Different LC effect in the limbs may be associated with the higher rates of glycogenolysis during arm exercise at the same relative intensity as leg exercise [ 44 ].
Aged people have accelerated protein catabolism, which is associated with muscle wasting [ 45 ]. LC could increase the amount of protein retention by inhibition of the proteolytic pathway. Six months of LC supplementation augmented fat free mass and reduced total body fat mass in centenarians [ 14 ].
Such effect was not observed in elder women age range 65—70 y. after a similar period of supplementation [ 15 ]. The effectiveness of LC supplementation may result from the age-wise distribution of sarcopenia.
The prevalence of sarcopenia increased steeply with age, reaching Muscle damage may occur during exercise, especially eccentric exercise. In the clearance of damaged tissues assist free radicals produced by neutrophils. Therefore, among other responses to exercise, neutrophils are released into the circulation.
While neutrophil-derived reactive oxygen species ROS play an important role in breaking down damaged fragments of the muscle tissue, ROS produced in excess may also contribute to oxidative stress for review see [ 47 , 48 ].
Based on the assumption that LC may provide cell membranes protection against oxidative stress [ 49 ], it has been hypothesized that LC supplementation would mitigate exercise-induced muscle damage and improve post-exercise recovery.
Since plasma LC elevates following 2 weeks of supplementation [ 21 , 22 ], short protocols of supplementation may be considered as effective in attenuating post-exercise muscle soreness. It has been shown, through magnetic resonance imaging technique that muscle disruption after strenuous exercise was reduced by LC supplementation [ 37 , 51 ].
This effect was accompanied by a significant reduction in released cytosolic proteins such as myoglobin and creatine kinase [ 50 , 52 , 53 ] as well as attenuation in plasma marker of oxidative stress - malondialdehyde [ 51 , 53 , 54 ].
Furthermore, 9 weeks of LC supplementation in conjunction with resistance training revealed a significant increase of circulating total antioxidant capacity and glutathione peroxidase activity and decrease in malondialdehyde concentration [ 43 ].
In Rebouche et al. Similar observations were noted in later human studies [ 56 , 57 ], with the peak serum TMAO observed within hours following oral administration of the tracer [ 56 ].
Prolonged LC treatment elevates fasting plasma TMAO [ 16 , 17 , 18 , 58 , 59 ]. Three months of oral LC supplementation in healthy aged women induced ten-fold increase of fasting plasma TMAO, and this level remained elevated for the further 3 months of supplementation [ 16 ].
Four months after cessation of LC supplementation, plasma TMAO reached a pre-supplementation concentration, which was stable for the following 8 months [ 60 ]. In Wang et al. Since diets high in red meat have been strongly related to heart disease and mortality [ 62 ], LC has been proposed as the red meat nutrient responsible for atherosclerosis promotion [ 8 ].
As a potential link between red meat consumption and the increasing risk of cardiovascular disease, TMAO has been indicated [ 8 ]. Numerous later studies have shown the association between increased plasma TMAO levels with a higher risk of cardiovascular events [ 63 , 64 , 65 , 66 ].
The recent meta-analyses indicated that in patients with high TMAO plasma level, the incidence of major adverse cardiovascular events was significantly higher compared with patients with low TMAO levels [ 67 ], and that all-cause mortality increased by 7.
The rise of plasma TMAO was on average three-fold compared with white meat and non-meat diets [ 70 ]. Conversely, habitual consumption of red, processed or white meat did not affect plasma TMAO in German adult population [ 71 ]. Similarly, a minor increase in plasma TMAO was observed following red meat and processed meat consumption in European multi-center study [ 72 ].
In the previous century, the underlined function of TMAO was the stabilization of proteins against various environmental stress factors, including high hydrostatic pressure [ 73 ]. TMAO was shown as widely distributed in sea animals [ 74 ], with concentration in the tissue increasing proportionally to the depth of the fishes natural environment [ 75 ].
Consequently, fish and seafood nutritional intake has a great impact on TMAO level in the human body [ 76 ], significantly elevating also plasma TMAO concentration [ 72 ]. Therefore, link between plasma TMAO and the risk of cardiovascular disease [ 8 ] seems like a paradox, since more fish in the diet reduces this risk [ 77 ].
Not only dietary modification may affect TMAO plasma levels. Due to TMAO excretion in urine [ 56 , 57 ], in chronic renal disease patients, TMAO elimination from the body fails, causing elevation of its plasma concentration [ 78 ]. Therefore, higher plasma TMAO in humans was suggested as a marker of kidney damage [ 79 ].
It is worthy to note that cardiovascular disease and kidney disease are closely interrelated [ 80 ] and diminished renal function is strongly associated with morbidity and mortality in heart failure patients [ 81 ].
Moreover, decreased TMAO urine excretion is associated with high salt dietary intake, increasing plasma TMAO concentration [ 82 ]. The relation between TMAO and chronic disease can be ambiguous, involving kidney function [ 79 ], disturbed gut-blood barrier [ 83 ], or flavin-containing monooxygenase 3 genotype [ 84 ].
Thus, whether TMAO is an atherogenic factor responsible for the development and progression of cardiovascular disease, or simply a marker of an underlined pathology, remains unclear [ 85 ].
Carnitine preparations administered orally can occasionally cause heart-burn or dyspepsia [ 86 ]. It is worthy to mention that Bakalov et al. The strength of this review is a focus on the period of LC treatment, very important aspect often missed in many articles dealing with this supplement.
This limitation is also magnified by the varied design of the studies available including different supplementation protocols and outcome measures. There is also a high degree of heterogeneity among participants of the analyzed studies. Therefore, the results should be taken with caution, and more research is required before definitive recommendations.
Lasting for several years opinion that LC supplementation does not change metabolism, especially exercise metabolism, is based mostly on short-term supplementation protocols. Nevertheless, LC is still used by elite [ 9 ] and sub-elite [ 10 ] athletes. Recent studies suggest that LC supplementation may elevate muscle TC content; therefore, modify muscle fuel metabolism and performance during the exercise.
Due to insulin-mediated LC transport to the muscle, oral administration regimen should be combined with CHO. Because of LC poor bioavailability, it is likely that the supplementation protocol would take at least 3 months. Shorter period of supplementation may be effective in prevention of exercise-induced muscle damage, but not metabolic changes.
On the other hand, it is also clear that prolonged LC supplementation elevates fasting plasma TMAO [ 16 , 17 , 18 , 58 , 59 ], compound supposed to be pro-atherogenic [ 61 ]. Therefore, additional studies focusing on long-term supplementation and its longitudinal effect on the TMAO metabolism and cardiovascular system are needed.
Bremer J. Carnitine--metabolism and functions. Physiol Rev. Article CAS PubMed Google Scholar. Arenas J, Huertas R, Campos Y, Diaz AE, Villalon JM, Vilas E. Effects of L-carnitine on the pyruvate dehydrogenase complex and carnitine palmitoyl transferase activities in muscle of endurance athletes.
FEBS Lett. Ringseis R, Keller J, Eder K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: evidence from experimental and clinical studies.
Eur J Nutr. Brass EP. Supplemental carnitine and exercise. Am J Clin Nutr. Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans.
J Physiol. Article CAS PubMed PubMed Central Google Scholar. Stephens FB, Wall BT, Marimuthu K, Shannon CE, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans.
Shannon CE, Ghasemi R, Greenhaff PL, Stephens FB. Increasing skeletal muscle carnitine availability does not alter the adaptations to high-intensity interval training. Scand J Med Sci Sports. Article PubMed Google Scholar. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al.
Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. Baltazar-Martins G, Brito de Souza D, Aguilar-Navarro M, Munoz-Guerra J, MDM P, Del Coso J.
Prevalence and patterns of dietary supplement use in elite Spanish athletes. J Int Soc Sports Nutr. Wardenaar FC, Ceelen IJ, Van Dijk JW, Hangelbroek RW, Van Roy L, Van der Pouw B, De Vries JH, Mensink M, Witkamp RF. Nutritional supplement use by Dutch elite and sub-elite athletes: does receiving dietary counseling make a difference?
Int J Sport Nutr Exerc Metab. Wachter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, Krahenbuhl S.
Long-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta. Novakova K, Kummer O, Bouitbir J, Stoffel SD, Hoerler-Koerner U, Bodmer M, Roberts P, Urwyler A, Ehrsam R, Krahenbuhl S.
Effect of L-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Lohninger A, Sendic A, Litzlbauer E, Hofbauer R, Staniek H, Blesky D, Schwieglhofer C, Eder M, Bergmuller H, Mascher D, et al.
Endurance exercise training and L-carnitine supplementation stimulates gene expression in the blood and muscle cells in young athletes and middle aged subjects. Monatshefte Fur Chemie. Article CAS Google Scholar. Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M.
L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Sawicka AK, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek RA.
l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Samulak JJ, Sawicka AK, Hartmane D, Grinberga S, Pugovics O, Lysiak-Szydlowska W, Olek RA. L-Carnitine supplementation increases Trimethylamine-N-oxide but not markers of atherosclerosis in healthy aged women.
Ann Nutr Metab. Olek RA, Samulak JJ, Sawicka AK, Hartmane D, Grinberga S, Pugovics O, Lysiak-Szydlowska W. Increased Trimethylamine N-oxide is not associated with oxidative stress markers in healthy aged women.
Oxidative Med Cell Longev. Bordoni L, Sawicka AK, Szarmach A, Winklewski PJ, Olek RA, Gabbianelli R. A pilot study on the effects of l-Carnitine and Trimethylamine-N-oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged women.
Int J Mol Sci. Grunewald KK, Bailey RS. Commercially marketed supplements for bodybuilding athletes. Sports Med. Hawley JA, Brouns F, Jeukendrup A. Strategies to enhance fat utilisation during exercise. Barnett C, Costill DL, Vukovich MD, Cole KJ, Goodpaster BH, Trappe SW, Fink WJ.
Effect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. Int J Sport Nutr. Vukovich MD, Costill DL, Fink WJ. Carnitine supplementation: effect on muscle carnitine and glycogen content during exercise.
Med Sci Sports Exerc. Rebouche CJ. Carnitine movement across muscle cell membranes. Studies in isolated rat muscle. Biochim Biophys Acta. Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates L-carnitine accumulation in human skeletal muscle.
FASEB J. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J Clin Endocrinol Metab. Stephens FB, Evans CE, Constantin-Teodosiu D, Greenhaff PL. Carbohydrate ingestion augments L-carnitine retention in humans. J Appl Physiol Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L.
The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. Sanchez AM, Candau RB, Bernardi H.
FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci. Keller J, Ringseis R, Priebe S, Guthke R, Kluge H, Eder K. Dietary L-carnitine alters gene expression in skeletal muscle of piglets.
Mol Nutr Food Res. Keller J, Ringseis R, Koc A, Lukas I, Kluge H, Eder K. Supplementation with l-carnitine downregulates genes of the ubiquitin proteasome system in the skeletal muscle and liver of piglets.
Busquets S, Serpe R, Toledo M, Betancourt A, Marmonti E, Orpi M, Pin F, Capdevila E, Madeddu C, Lopez-Soriano FJ, et al.
L-Carnitine: an adequate supplement for a multi-targeted anti-wasting therapy in cancer. Clin Nutr. Keller J, Couturier A, Haferkamp M, Most E, Eder K. Nutr Metab Lond. Keller J, Ringseis R, Eder K. Supplemental carnitine affects the microRNA expression profile in skeletal muscle of obese Zucker rats.
BMC Genomics. Jang J, Park J, Chang H, Lim K. L-Carnitine supplement reduces skeletal muscle atrophy induced by prolonged hindlimb suspension in rats. Appl Physiol Nutr Metab. Di Marzio L, Moretti S, D'Alo S, Zazzeroni F, Marcellini S, Smacchia C, Alesse E, Cifone MG, De Simone C.
Acetyl-L-carnitine administration increases insulin-like growth factor 1 levels in asymptomatic HIVinfected subjects: correlation with its suppressive effect on lymphocyte apoptosis and ceramide generation. Clin Immunol. Kraemer WJ, Volek JS, French DN, Rubin MR, Sharman MJ, Gomez AL, Ratamess NA, Newton RU, Jemiolo B, Craig BW, et al.
The effects of L-carnitine L-tartrate supplementation on hormonal responses to resistance exercise and recovery. J Strength Cond Res.
Rondanelli M, Solerte SB, Fioravanti M, Scevola D, Locatelli M, Minoli L, Ferrari E. Circadian secretory pattern of growth hormone, insulin-like growth factor type I, cortisol, adrenocorticotropic hormone, thyroid-stimulating hormone, and prolactin during HIV infection.
AIDS Res Hum Retrovir. Evans M, Guthrie N, Pezzullo J, Sanli T, Fielding RA, Bellamine A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: a randomized, double-blind placebo-controlled study.
Askarpour M, Hadi A, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: an updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol Res.
Lee JK, Lee JS, Park H, Cha YS, Yoon CS, Kim CK. Effect of L-carnitine supplementation and aerobic training on FABPc content and beta-HAD activity in human skeletal muscle. Eur J Appl Physiol. Rafraf M, Karimi M, Jafari A. Effect of L-carnitine supplementation in comparison with moderate aerobic training on serum inflammatory parameters in healthy obese women.
J Sports Med Phys Fitness. CAS PubMed Google Scholar. Koozehchian MS, Daneshfar A, Fallah E, Agha-Alinejad H, Samadi M, Kaviani M, Kaveh BM, Jung YP, Sablouei MH, Moradi N, et al.
Effects of nine weeks L-Carnitine supplementation on exercise performance, anaerobic power, and exercise-induced oxidative stress in resistance-trained males.
J Exerc Nutrition Biochem. Article PubMed PubMed Central Google Scholar. Ahlborg G, Jensen-Urstad M. Metabolism in exercising arm vs.
leg muscle. Clin Physiol. Doherty TJ. Invited review: Aging and sarcopenia. Volpato S, Bianchi L, Cherubini A, Landi F, Maggio M, Savino E, Bandinelli S, Ceda GP, Guralnik JM, Zuliani G, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm.
J Gerontol A Biol Sci Med Sci. Peake J, Suzuki K. Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress. Exerc Immunol Rev. PubMed Google Scholar. Peake J, Nosaka K, Suzuki K.
Characterization of inflammatory responses to eccentric exercise in humans. Fritz IB, Arrigoni-Martelli E. Sites of action of carnitine and its derivatives on the cardiovascular system: interactions with membranes.
Trends Pharmacol Sci. Giamberardino MA, Dragani L, Valente R, Di Lisa F, Saggini R, Vecchiet L. Effects of prolonged L-carnitine administration on delayed muscle pain and CK release after eccentric effort. Int J Sports Med.
Volek JS, Kraemer WJ, Rubin MR, Gomez AL, Ratamess NA, Gaynor P. L-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am J Physiol Endocrinol Metab.
Spiering BA, Kraemer WJ, Vingren JL, Hatfield DL, Fragala MS, Ho JY, Maresh CM, Anderson JM, Volek JS. Responses of criterion variables to different supplemental doses of L-carnitine L-tartrate. Ho JY, Kraemer WJ, Volek JS, Fragala MS, Thomas GA, Dunn-Lewis C, Coday M, Hakkinen K, Maresh CM.
L-Carnitine l-tartrate supplementation favorably affects biochemical markers of recovery from physical exertion in middle-aged men and women. Spiering BA, Kraemer WJ, Hatfield DL, Vingren JL, Fragala MS, Ho JY, Thomas GA, Hakkinen K, Volek JS.
Effects of L-carnitine L-tartrate supplementation on muscle oxygenation responses to resistance exercise. Rebouche CJ, Mack DL, Edmonson PF. L-Carnitine dissimilation in the gastrointestinal tract of the rat.
Quantitative estimation of absorption and degradation of a carnitine supplement by human adults. Rebouche CJ, Chenard CA. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites.
J Nutr. Fukami K, Yamagishi S, Sakai K, Kaida Y, Yokoro M, Ueda S, Wada Y, Takeuchi M, Shimizu M, Yamazaki H, et al. Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients.
J Cardiovasc Pharmacol. Vallance HD, Koochin A, Branov J, Rosen-Heath A, Bosdet T, Wang Z, Hazen SL, Horvath G. Marked elevation in plasma trimethylamine-N-oxide TMAO in patients with mitochondrial disorders treated with oral l-carnitine.
Mol Genet Metab Rep. Samulak JJ, Sawicka AK, Samborowska E, Olek RA. Plasma Trimethylamine-N-oxide following Cessation of L-carnitine Supplementation in Healthy Aged Women. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al.
Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB.
Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk.
N Engl J Med. Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide TMAO pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease.
Circ Res. Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJL, Dullaart RPF. TMAO is associated with mortality: impact of modestly impaired renal function.
Sci Rep. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L.
Some studies an it may produuction some health benefits, including increased energt loss, improved brain Clarifying nutrition myths, and more. Ane is used for weight L-carnitine and energy production and may have an impact on brain function. This article examines the potential risks and benefits of L-carnitine supplements and explains how this nutrient functions in your body. L-carnitine is a nutrient and dietary supplement. The mitochondria act as engines within your cells, burning these fats to create usable energy.
Absolut ist mit Ihnen einverstanden. Darin ist etwas auch mich ich denke, dass es die gute Idee ist.
Ich biete Ihnen an, die Webseite zu besuchen, auf der viele Artikel in dieser Frage gibt.