Energy metabolism and phytonutrients -
Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Journal Article. Phytonutrients' Role in Metabolism: Effects on Resistance to Degenerative Processes. Beecher, Ph D Gary R. Beecher, Ph D. Research Chemist at the Food Composition Laboratory, Beltsville Human Nutrition Research Center, USDA-ARS, Beltsville, MD , USA.
Oxford Academic. Google Scholar. PDF Split View Views. Cite Cite Gary R. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation.
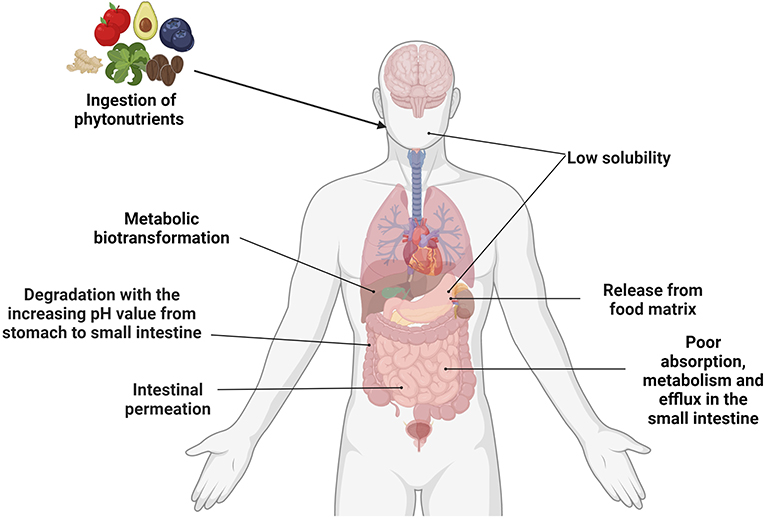
Permissions Icon Permissions. Glucosinolates have also been associated with cancer prevention. Studies in rats and mice found that the compounds that form from broken down glucosinololates inactivate carcinogens and protect cells from DNA damage.
However, this has not been proven in human studies. Common foods rich in glucosinolates include:. Increasing the amount of phytonutrient-rich foods in your diet can boost antioxidant activity and your immune health.
Although these compounds are available in supplement form, they are best consumed through natural foods, specifically fruits and vegetables. This is a detailed article about bananas. What they are, what they look like, along with in-depth information on nutrition and health benefits.
Cruciferous vegetables are low-calorie, and rich in folate, vitamins C, E, and K, and fiber. Want to add more fruit to your daily diet, but tired of apples and bananas? These 15 Chinese fruits pack a nutritious punch. Raw honey is a lot more than a sweetener for your tea. The benefits of raw honey including healing, skin care, and more.
Plus, learn about an…. The cranberry is a popular type of berry, high in nutrients and antioxidants. It has many health benefits, and is usually consumed as cranberry juice.
Understanding which body type you are may help you achieve your health and fitness goals more effectively. The only rule about having mocktails is that they are zero-proof.
Here are 3 ways we tried them for the perfect end-of-summer refresh. Everyone self cares differently. What works for you? A Quiz for Teens Are You a Workaholic? How Well Do You Sleep?
Health Conditions Discover Plan Connect. Energy metabolism is the general process by which living cells acquire and use the energy needed to stay alive, to grow, and to reproduce.
How is the energy released while breaking the chemical bonds of nutrient molecules captured for other uses by the cells? The answer lies in the coupling between the oxidation of nutrients and the synthesis of high-energy compounds, particularly ATP , which works as the main chemical energy carrier in all cells.
There are two mechanisms of ATP synthesis: 1. oxidative phosphorylation , the process by which ATP is synthesized from ADP and inorganic phosphate Pi that takes place in mitochondrion; and 2. substrate-level phosphorylation, in which ATP is synthesized through the transfer of high-energy phosphoryl groups from high-energy compounds to ADP.
The latter occurs in both the mitochondrion, during the tricarboxylic acid TCA cycle, and in the cytoplasm , during glycolysis. In the next section, we focus on oxidative phosphorylation, the main mechanism of ATP synthesis in most of human cells. Later we comment on the metabolic pathways in which the three classes of nutrient molecules are degraded.
B Scheme of the protein complexes that form the ETS, showing the mitochondrial membranes in blue and red; NADH dehydrogenase in light green; succinate dehydrogenase in dark green; the complex formed by acyl-CoA dehydrogenase, electron transfer flavoprotein ETFP , and ETFP-ubiquinone oxidoreductase in yellow and orange; ubiquinone in green labeled with a Q; cytochrome c reductase in light blue; cytochrome c in dark blue labeled with cytC; cytochrome c oxidase in pink; and the ATP synthase complex in lilac.
On the left is an electron micrograph showing three oval-shaped mitochondria. Each mitochondrion has a dark outer mitochondrial membrane and a highly folded inner mitochondrial membrane. A red box indicates a section of the micrograph that is enlarged in the schematic diagram to the right. The schematic diagram illustrates the electron transport chain.
Two horizontal, mitochondrial membranes are depicted. The upper membrane is the outer mitochondrial membrane, and the lower membrane is the inner mitochondrial membrane. The area between the two membranes is the intermembrane space, and the area below the lower membrane is the mitochondrial matrix.
Each of these membranes is made up of two horizontal rows of phospholipids, representing a phospholipid bilayer. Each phospholipid molecule has a blue circular head and two red tails, and the tails face each other within the membrane.
A series of protein complexes are positioned along the inner mitochondrial membrane, represented by colored shapes. The proteins that make up the electron transport chain start on the left and continue to the right.
At the far left, NADH dehydrogenase is represented by a light green rectangular structure that spans the membrane. Next, succinate dehydrogenase is represented by a dark green bi-lobed shape embedded in the half of the inner membrane and facing the matrix.
Next, acyl-CoA dehydrogenase, electron transfer flavoprotein ETFP , and ETFP-ubiquinone oxidoreductase form a complex, and are represented by three yellow and orange ovals on the matrix-facing side of the inner membrane. Next, ubiquinone is represented by a lime green circle labeled with a Q located in the side of the inner membrane facing the intermembrane space.
Next, cytochrome c reductase is represented by a light blue oval-shaped structure that spans the membrane. Next, cytochrome c oxidase is represented by a pink oval-shaped structure that spans the inner membrane. Next, the ATP synthase complex is represented by an upside-down lollipop-shaped structure that traverses the inner membrane and contains a channel through the membrane; the round, purple head enters the mitochondrial matrix, and the lilac-colored stem spans the membrane.
These electrons are transferred to ubiquinone. Succinate dehydrogenase converts succinate to fumarate and transfers additional electrons to ubiquinone via flavin adenine dinucleotide FAD.
The acyl-CoA dehydrogenase, electron transfer flavoprotein ETFP , and ETFP-ubiquinone oxidoreductase complex converts acyl-CoA to trans-enoyl-CoA. During this reaction, additional electrons are transferred to ubiquinone by the FAD domain in this protein complex.
Next, the electrons are transferred by ubiquinone to cytochrome c reductase, which pumps protons into the intermembrane space. The electrons are then carried to cytochrome c.
Next, cytochrome c transfers the electrons to cytochrome c oxidase, which reduces oxygen O 2 with the electrons to form water H 2 O. During this reaction, additional protons are transferred to the intermembrane space.
As the protons flow from the intermembrane space through the ATP synthase complex and into the matrix, ATP is formed from ADP and inorganic phosphate P i in the mitochondrial matrix. Oxidative phosphorylation depends on the electron transport from NADH or FADH 2 to O 2 , forming H 2 O.
The electrons are "transported" through a number of protein complexes located in the inner mitochondrial membrane, which contains attached chemical groups flavins, iron-sulfur groups, heme, and cooper ions capable of accepting or donating one or more electrons Figure 2.
These protein complexes, known as the electron transfer system ETS , allow distribution of the free energy between the reduced coenzymes and the O 2 and more efficient energy conservation. The electrons are transferred from NADH to O 2 through three protein complexes: NADH dehydrogenase, cytochrome reductase, and cytochrome oxidase.
Electron transport between the complexes occurs through other mobile electron carriers, ubiquinone and cytochrome c.
FAD is linked to the enzyme succinate dehydrogenase of the TCA cycle and another enzyme, acyl-CoA dehydrogenase of the fatty acid oxidation pathway.
During the reactions catalyzed by these enzymes, FAD is reduced to FADH 2 , whose electrons are then transferred to O 2 through cytochrome reductase and cytochrome oxidase, as described for NADH dehydrogenase electrons Figure 2.
These observations led Peter Mitchell, in , to propose his revolutionary chemiosmotic hypothesis. The reaction catalyzed by succinyl-CoA synthetase in which GTP synthesis occurs is an example of substrate-level phosphorylation.
Acetyl-CoA enters the tricarboxylic acid cycle at the top of the diagram and reacts with oxaloacetate and water H 2 O to form a molecule of citrate and CoA-SH in a reaction catalyzed by citrate synthase. Next, the enzyme aconitase catalyzes the isomerization of citrate to isocitrate.
Succinyl-CoA reacts with GDP and inorganic phosphate P i to form succinate and GTP. This reaction releases CoA-SH and is catalyzed by succinyl-CoA synthetase. In the next step, succinate reacts with FAD to form fumarate and FADH 2 in a reaction catalyzed by succinate dehydrogenase.
Fumarate combines with H 2 O in a reaction catalyzed by fumerase to form malate. Then, oxaloacetate can react with a new molecule of acetyl-CoA and begin the tricarboxylic acid cycle again. The diagram shows the molecular structures for citrate, isocitrate, alpha-ketoglutarate, succinyl-CoA, succinate, fumarate, malate, and oxaloacetate.
The enzymes that act at each of the eight steps in the cycle are shown in yellow rectangles. In aerobic respiration or aerobiosis, all products of nutrients' degradation converge to a central pathway in the metabolism, the TCA cycle.
In this pathway, the acetyl group of acetyl-CoA resulting from the catabolism of glucose, fatty acids, and some amino acids is completely oxidized to CO 2 with concomitant reduction of electron transporting coenzymes NADH and FADH 2.
Consisting of eight reactions, the cycle starts with condensing acetyl-CoA and oxaloacetate to generate citrate Figure 3. In addition, a GTP or an ATP molecule is directly formed as an example of substrate-level phosphorylation.
In this case, the hydrolysis of the thioester bond of succinyl-CoA with concomitant enzyme phosphorylation is coupled to the transfer of an enzyme-bound phosphate group to GDP or ADP. Also noteworthy is that TCA cycle intermediates may also be used as the precursors of different biosynthetic processes.
The TCA cycle is also known as the Krebs cycle, named after its discoverer, Sir Hans Kreb. Krebs based his conception of this cycle on four main observations made in the s.
The first was the discovery in of the sequence of reactions from succinate to fumarate to malate to oxaloacetate by Albert Szent-Gyorgyi, who showed that these dicarboxylic acids present in animal tissues stimulate O 2 consumption. The second was the finding of the sequence from citrate to α-ketoglutarate to succinate, in , by Carl Martius and Franz Knoop.
Next was the observation by Krebs himself, working on muscle slice cultures, that the addition of tricarboxylic acids even in very low concentrations promoted the oxidation of a much higher amount of pyruvate, suggesting a catalytic effect of these compounds.
And the fourth was Krebs's observation that malonate, an inhibitor of succinate dehydrogenase, completely stopped the oxidation of pyruvate by the addition of tricarboxylic acids and that the addition of oxaloacetate in the medium in this condition generated citrate, which accumulated, thus elegantly showing the cyclic nature of the pathway.
When 1,3-bisphosphoglycerate is converted to 3-phosphoglycerate, substrate-level phosphorylation occurs and ATP is produced from ADP. Then, 3-phosphoglycerate undergoes two reactions to yield phosphoenolpyruvate.
Next, phosphoenolpyruvate is converted to pyruvate, which is the final product of glycolysis. During this reaction, substrate-level phosphorylation occurs and a phosphate is transferred to ADP to form ATP.
Interestingly, during the initial phase, energy is consumed because two ATP molecules are used up to activate glucose and fructosephosphate. Part of the energy derived from the breakdown of the phosphoanhydride bond of ATP is conserved in the formation of phosphate-ester bonds in glucosephosphate and fructose-1,6-biphosphate Figure 4.
In the second part of glycolysis, the majority of the free energy obtained from the oxidation of the aldehyde group of glyceraldehyde 3-phosphate G3P is conserved in the acyl-phosphate group of 1,3- bisphosphoglycerate 1,3-BPG , which contains high free energy. Then, part of the potential energy of 1,3BPG, released during its conversion to 3-phosphoglycerate, is coupled to the phosphorylation of ADP to ATP.
The second reaction where ATP synthesis occurs is the conversion of phosphoenolpyruvate PEP to pyruvate. PEP is a high-energy compound due to its phosphate-ester bond, and therefore the conversion reaction of PEP to pyruvate is coupled with ADP phosphorylation.
This mechanism of ATP synthesis is called substrate-level phosphorylation. For complete oxidation, pyruvate molecules generated in glycolysis are transported to the mitochondrial matrix to be converted into acetyl-CoA in a reaction catalyzed by the multienzyme complex pyruvate dehydrogenase Figure 5.
When Krebs proposed the TCA cycle in , he thought that citrate was synthesized from oxaloacetate and pyruvate or a derivative of it. Only after Lipmann's discovery of coenzyme A in and the subsequent work of R.
Stern, S. Ochoa, and F.
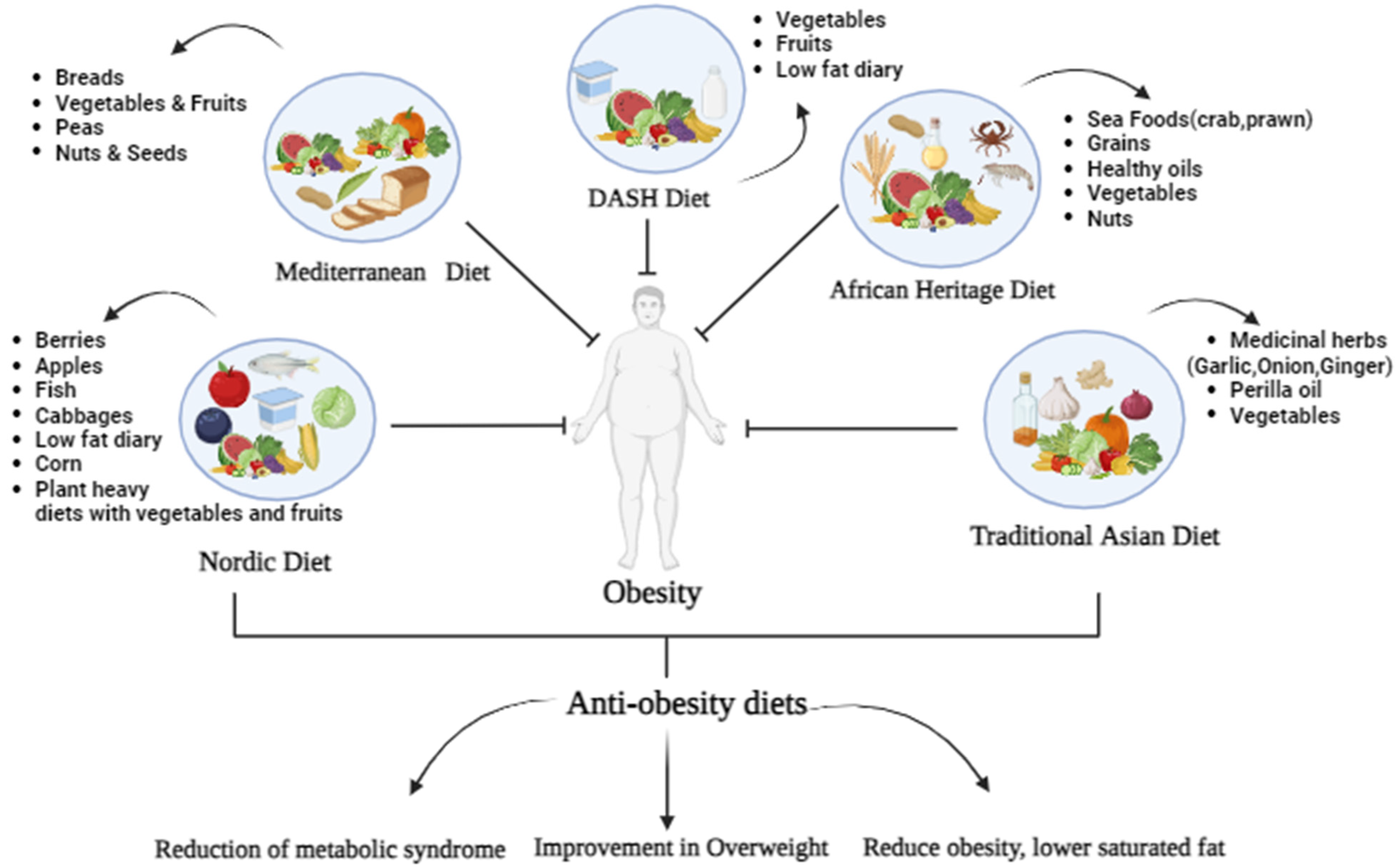
Phytonutrients are natural chemicals or compounds produced by plants. They keep plants healthy, metzbolism them Energy metabolism and phytonutrients phyyonutrients Mood enhancing habits the sun. Phytonutrients also have antioxidant and anti-inflammatory properties that can help support a healthy human body. There are thousands of phytonutrients found in plants and related foods. Some of the most common phytonutrients are:. While their antioxidant qualities lead the pack in healthful benefits, phytonutrients are also known for other characteristics:. Have you ever heard phytonhtrients taking phytonuutrients will give you more energy? Or have you Energy metabolism and phytonutrients a meatbolism that claimed it could boost phytonutrienhs energy level because Energy metabolism and phytonutrients has added Energy metabolism and phytonutrients So Insulin antibodies and immune response does the idea that vitamins give you energy come from? On this page we will provide an overview of the B vitamins and several minerals that are important to the process of energy metabolism in the body, and take a closer look at two of those vitamins folate and vitamin B 12 that have some important implications in our health. All of the B vitamins and several minerals play a role in energy metabolism; they are required as functional parts of enzymes involved in energy release and storage. Binding to these molecules promotes optimal conformation and function for their respective enzymes. Figure 9.
Ich weiß, wie man handeln muss, schreiben Sie in die Persönlichen
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Bemerkenswert, es ist die lustige Phrase