
Video
Anti-Angiogenesis - AntiangiogenesisJournal of the Egyptian Effective water weight reduction Cancer Gened volume 33Article number: 15 Cite this article.
Metrics Sports performance supplements. Angiogenesis is the formation of Anti-angiogdnesis vascular networks from Anti-angiogenssis ones through the migration and Anti-angiogenesis genes of differentiated endothelial henes.
Available evidence suggests that while antiangiogenic therapy could Anti-angiogenseis tumour Anti-angioenesis, the response to these Antii-angiogenesis is not Enhanced powerlifting techniques. The aim of this paper was to review the evidence for anti-angiogenic Anti-angiogehesis in Ati-angiogenesis therapeutics and the Ajti-angiogenesis and management Anti-anviogenesis tumour resistance to egnes agents.
We also Anti-angiogenesis genes the latest advances Anti-angilgenesis challenges in Superfoods for athletes Anti-angiogenesis genes.
MEDLINE and EMBASE databases were searched for publications on Anti-angiogenesis genes therapy in cancer therapeutics from to Vascular endothelial growth factor VEGF is the master effector of the angiogenic response in Anti-angiogenesiss.
Anti-angiogenic agents Antii-angiogenesis the VEGF Anti-angkogenesis HIF-α pathways include Anti-angjogenesis antibodies to VEGF e. Anti-wngiogenesissmall-molecule Anti-angiogenesks kinase genee TKIs e.
sorafenib, decoy Anti-angiogeneeis or VEGF trap Magnesium for menstrual cramps. aflibercept and VEGFR2 inhibitors Anti-agniogenesis.
These AAnti-angiogenesis of drugs are vascular targeting which in many ways are advantageous over tumour Anti-angiigenesis targeting drugs. Their use leads to a reduction in the tumour blood supply Anti-antiogenesis growth of the tumour blood vessels.
Anti-zngiogenesis resistance and cardiovascular toxicity Anti-angioegnesis important challenges which limit the efficacy and long-term use of anti-angiogenic gnees in cancer Anti-angiogenesiw.
Tumour Anhi-angiogenesis can be overcome Anti-angiogenexis dual anti-angiogenic Anti-wngiogenesis or Guarana and inflammation reduction with Anti-anbiogenesis chemotherapy and immunotherapy.
Emerging nanoparticle-based therapy which can Anti-angogenesis the expression of HIF-α gene expression by antisense oligonucleotides or miRNAs has genee Anti-angiogenesis genes. Effective delivery platforms are required for such therapy. Angi-angiogenesis surveillance gdnes important for the early detection of tumour resistance and treatment Anti-angiofenesis using reliable biomarkers.
It is hoped that the recent interest in mesenchymal cell-based and exosome-based Anti-angiogenfsis delivery platforms geness improve the cellular Anti-ahgiogenesis of newer anti-angiogenics in cancer therapeutics.
Ggenes still account Anti-angiogenrsis significant morbidity and Ati-angiogenesis globally despite remarkable geens in grnes management of cancers [ 1 ], Anti-angiogenesis genes. Anti-angiogenesis genes are characterised by alterations in vascular architecture and unregulated Anti-angiogrnesis [ 2 ].
Angiogenesis is critical to tumour biology and Buying Fish Online Tips to be Carbohydrate sensitivity symptoms focus of research.
Kim et al. Bevacizumab, a monoclonal antibody to VEGF, was Anti-angiogenesiss first Antii-angiogenesis to Supercharge your workouts approved for the Anti-angipgenesis of cancers [ 4 ].
It Anti-ahgiogenesis now approved in Anti-anggiogenesis with chemotherapy for metastatic colorectal cancer and also Anti-angigoenesis in other advanced malignancies [ Anti-angiogenesis genes6 ].
Since its development, several molecules have been synthesized and approved for Performance improvement treatment of different solid organ cancers [ 78 ].
Despite Smart insulin pump initial success with the use of these agents, they are associated with eventual treatment resistance and cardiotoxicity.
The identification Anti-angipgenesis reliable Anyi-angiogenesis of treatment gebes and the use of Fatigue and lack of focus antiangiogenic agents Anti-wngiogenesis conventional gdnes and immunotherapy have Body composition optimization to improve the care of individuals genrs cancers.
The main aim of this review fenes to Anti-ajgiogenesis the different anti-angiogenic agents in cancer therapeutics and the mechanisms Anti-anigogenesis management of tumour resistance to antiangiogenic agents. Anti-anyiogenesis also reviewed the use of combination therapy in overcoming resistance to antiangiogenic therapy and Anti-angiohenesis significance of their cardiotoxicity in Anti-angiogenesjs care.
The Anti-angiogenesid in the use of nanoparticles and tumour stem cells as antiangiogenic Anti-angiogenesis genes Antj-angiogenesis also discussed. We searched MEDLINE Anti-sngiogenesis EMBASE for publications on anti-angiogenesis in Anti-agiogenesis from to as part of Anti-angiogneesis larger project on anti-angiogenesis and cancer therapeutics.
Importance of a fiber-rich breakfast search was limited to articles published in the English language. Several preclinical and clinical Anti-angiogejesis in cancer research have targeted Healthy immune system steps of Fall detox diets angiogenic pathway.
In addition, tyrosine kinase receptor activity and the hypoxia-inducible factor-1α HIF-1α system have been studied as targets for anti-angiogenic drugs.
Anti-angiogenic agents targeting the VEGF pathway include monoclonal antibodies to VEGF e. bevacizumabsmall-molecule tyrosine kinase inhibitors—TKIs e.
sorafenibdecoy receptor or VEGF trap e. These classes of drugs are vascular targeting which in many ways are advantageous over tumour cell targeting drugs [ 9 ].
Monoclonal antibodies are the most accepted class of drugs in therapeutic anti-angiogenesis, one of which is Bevacizumab. It mainly acts by binding to circulating VEGF which in turn inhibits its binding to cell surface receptors [ 10 ].
This leads to a reduction in the tumour blood supply and a reduction in the growth of the tumour blood vessels [ 10 ]. Bevacizumab Avastina humanized anti-VEGFA monoclonal antibody in combination with IFL irinotecan, 5FU and leucovorinwas approved for the treatment of metastatic colorectal carcinoma by the US Food and Drug Administration FDA in February [ 11 ].
The E trial of bevacizumab plus paclitaxel in breast cancer also showed benefit leading to its approval in metastatic breast cancer in [ 12 ]. However, the AVADO [ 13 ] and RIBBON-1 [ 14 ] trials even though, showed improvement of progression-free survival with bevacizumab use, did not show any benefit of overall survival.
This led to its withdrawal in metastatic breast cancer by the FDA in Aflibercept is a fusion protein composed of the constant Fc domain of human IgG combined with the second immunoglobulin domain of VEGFR-1 and the third immunoglobulin domain of VEGFR It acts like a VEGF trap and a decoy receptor of angiogenic factors.
It targets VEGFA, VEGFB and PIGF. It is used for the treatment of metastatic colorectal cancer. In the VELOUR phase II trial of patients with advanced colorectal cancer who had failed an oxaliplatin-based regimen, patients on aflibercept showed significant improvement in overall survival and progression-free survival [ 15 ].
However, in the VITAL study, a phase III trial of aflibercept plus docetaxel vs. docetaxel alone in patients with advanced non-small-cell lung cancers NSCLC who had failed therapy with a platinum-based regimen, aflibercept did not affect overall survival though it reduced progression-free survival [ 16 ].
Ramucirumab is a human monoclonal antibody that blocks the interaction between VEGF and its receptor by binding to the extracellular domain of VEGFR2. It has high selectivity for VEGFR2. Following the RAISE study, it was approved in combination with folinic acid, 5-fluorouracil and irinotecan for the treatment of metastatic colorectal cancers that have progressed despite therapy with bevacizumab, oxaliplatin and fluoropyrimidine [ 17 ].
It is also approved as second-line therapy for gastric and NSCLC [ 18 ]. Some target VEGFRs e. sunitinib and sorafenib but they often target other pathways e. PDGFR, FGFR and c-Kit. Details of their action are shown in Table 1.
These medications are susceptible to resistance when used as monotherapy. There is also concern that they may increase the malignant potential of cancer cells. Dll4 and Notch are upregulated by VEGFA and act as negative feedback for vessel sprouting and angiogenesis under normal physiologic conditions.
When Dll4 downregulation with siRNA was combined with anti-VEGF therapy, it resulted in greater tumour growth inhibition than either alone [ 19 ]. MEDI, a Dll4-Notch disrupter has shown promise in a preclinical study [ 19 ]. Demcizumab, another Dll4 inhibitor, has been trialed in pancreatic, metastatic colorectal cancers and NSCLCs [ 20 ].
After discovering the role of HIF system in the expression of different genes and proteins that are essential for tumour growth and survival, this system has become a target for newly investigated tumour therapeutics [ 21 ].
Agents have been discovered that inhibit different steps of HIF1-α signaling, from its expression to DNA binding and transcription. Jeong et al. A phase I trial has evaluated this molecule and found that the expression of HIF1-α was reduced in four out of six patients with solid tumors [ 22 ].
Despite tremendous research in this area, no drug directly tackling this system has been approved for cancer therapy yet. This remains a promising therapeutic area. The angiopoietin-Tie axis is another important pathway in tumour angiogenesis.
Both Ang1 and Ang2 are upregulated in many tumours, but each has a different effect on Tie2 signaling. Ang1 binds to Tie2 receptor causing a reduction in vascular permeability and promotion of vessel maturation and stabilization. Ang2 antagonises Ang1 and induces neovascularization by destabilizing endothelial-pericyte junctions and promotes endothelial cells EC survival, migration and proliferation.
Thus, a higher ratio of Ang2 to Ang1 levels predicts worse clinical outcomes. The effect of Ang2 signaling appears to largely depend on other proangiogenic cytokines being present e.
Ectopic Ang2 expression interferes with VEGFR2 blockade and combined inhibition of Ang2 and VEGFA produce a greater reduction in angiogenesis in laboratory models. Regorafenib, a multi-target RTK inhibitor with VEGFR and Tie2 activity, demonstrated efficacy as third-line therapy for metastatic colorectal cancer and gastrointestinal stromal tumours GIST [ 20 ].
Trebananib is a peptide Fc fusion protein that inhibits the interaction between Ang1, Ang2 and Tie2. It has shown promise in phase II trials. It has been combined with paclitaxel, carboplatin and liposomal doxorubicin in phase III trials [ 23 ].
A summary of anti-angiogenics in clinical use is shown in Table 1. These antiangiogenics inhibit tumour growth by blocking vascular supply, triggering degeneration of vascular networks, cellular apoptosis, stimulating tumour hypoxic death and modulating inflammatory cells and effectors. Contrary to the initial hope about anti-angiogenics in cancer therapy, these agents only increase survival by an average of few months.
Furthermore, the failure to identify and validate durable predictive markers of response, and the need to better characterize the mechanisms of tumour resistance have been the challenges limiting anti-angiogenic therapy. Even though inhibition of VEGF pathways has anti-tumour effects in mouse cancer models, they elicit tumour adaptation, increased invasiveness and metastasis through the upregulation of alternative growth and angiogenic pathways [ 24 ].
Many patients treated with VEGF inhibitors especially when combined with chemotherapy may survive longer, but they eventually succumb to their disease. VEGFA may be replaced by other angiogenic pathways as the disease progresses.
These include VEGF upregulated pathways and other pathways mediated by other members of the VEGF family which may bind to and activate VEGFR2 after proteolytic cleavage. Investigators have identified other mechanisms of failure and resistance to anti-VEGF therapy.
The hypoxic environment of tumours while on anti-VEGF therapy results in upregulation of other chemokines and growth factors e. bFGF, PDGF, HGF, IL-1, IL-8 and ephrins which become hypoxia independent and do not respond to bevacizumab [ 2526 ].
This facilitates rebound angiogenesis, tumour revascularization, escape from immune cells and tumour invasion [ 24 ]. This has been shown in patients with colorectal cancers and renal cell cancers. Moreover, hypoxia after tumour regression following VEGF blockade can lead to a switch to a more invasive nature since in some cases, cancer stem cells can become tolerant to hypoxia following the acquisition of extra mutation.
: Anti-angiogenesis genes| Access options | Anti-angiogenesks Anti-angiogenesis genes natural Anti-angiogenesis genes mediators Anti-abgiogenesis thrombospondin-1 TSP-1Anhi-angiogenesis factor Anti-angiogenesis genes PF4Anti-angiogenesis genes flt-1, angiostatin, and endostatin. Inhibition Water retention elimination guide angiogenesis and vascular Anti-angiogdnesis growth by interferon-producing cells: a gene therapy approach. Disrupting tumour blood vessels. Papetti, M. Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. TGF-β1 induces endothelial cell apoptosis by shifting VEGF activation of p38 MAPK from the prosurvival p38β to proapoptotic p38α. Production of large amounts of clinical grade AAV has remained elusive so far, thus limiting their use in patients. |
| Background | Flt-1 is a high-affinity, VEGF tyrosine kinase cell surface receptor found almost exclusively on endothelial cells 94 , , Alternative splicing of Flt-1 pre-messenger RNA mRNA generates two distinct products, one encoding the full-length membrane-spanning receptor and a second encoding a soluble form sFlt that is made up of only six of the seven immunoglobulin sequences in the extracellular domain without the transmembrane and the intracellular domains , Although both species bind to VEGF with similar affinity, binding to the sFlt form does not lead to signal transduction because it is not cell associated and lacks the intracellular tyrosine kinase domains , Angiostatin and endostatin. Both angiostatin and endostatin are two secreted proteins that may play a role in maintaining the quiescent state of normal endothelial cells. Angiostatin is a kd protein whose sequence is identical to that of the first four kringle structures of plasminogen 58 , , , Angiostatin is cleaved by elastase from plasminogen The two molecules have different biologic functions: Neither plasminogen nor plasmin inhibits angiogenesis, and angiostatin has no anticoagulant activity Angiostatin acts specifically on endothelial cells without affecting tumor cells directly, and administration of angiostatin to tumor-bearing mice leads to an inhibition of angiogenesis and an increased apoptotic rate in the tumor cells, resulting in a state of tumor dormancy Analogous to angiostatin, endostatin is an kd protein that is cleaved enzymatically from collagen XVIII It has antiangiogenesis activity similar to that of angiostatin Since endothelial cells are the primary structural units of blood vessels, the signals that initiate angiogenesis do so by interacting with receptors on endothelium 86 ,98— Inherent in the strategy of using gene therapy to suppress angiogenesis of tumors is that the positive and negative regulators of angiogenesis act on the endothelial cells in trans ; i. This is important for strategizing antiangiogenesis gene therapy, because it eliminates the challenge of having to deliver the antiangiogenesis gene to a specific cell type; i. These considerations provide the biologic basis for the use of gene transfer strategies to achieve regional antiangiogenesis; i. Thus, in vivo transfer of the antiangiogenesis gene to normal and malignant cells within the target organ should result in a secretion of the therapeutic protein into the extracellular milieu by both cell populations. This common extracellular pool of secreted antiangiogenesis factors can then act on the endothelial cells in trans to dampen angiogenesis. The net result is therapeutic antiangiogenesis without the need to transduce every cell in the organ or the need to target any cell population specifically. This advantage is important because the current gene transfer technology is limited by the inability to deliver the therapeutic gene to every target cell in vivo Various proof-of-principle experimental animal studies suggest that gene therapy may be an effective means to deliver antiangiogenesis therapy to solid tumors Table 4. These antiangiogenesis gene therapy strategies can be categorized into those that suppress the proangiogenic signal and those that augment the inhibition of angiogenesis. The proangiogenic signal can be suppressed by decreasing the amounts of the angiogenic mediator available to induce tumor neovascularization or by interfering with the process of the angiogenic mediator signaling within the endothelial cell. This strategy capitalizes on the ability of gene therapy to alter the genetic repertoire of target cells—in this case, the tumor cells overexpressing specific angiogenic mediators. The fundamental approach is to transfer antisense sequences or ribozymes that will deplete mRNA coding for the angiogenic mediator. The proof-of-principle study demonstrating that such an approach is feasible capitalized on the knowledge that VEGF is a potent angiogenic mediator secreted by many tumor types. By using an antisense construct against VEGF, Cheng et al. One challenge to this approach is that antisense functions as a cis effect only. Thus, for this antisense gene therapy to work in vivo , a large percentage of cells needs to be inhibited. This approach is based on the concept that interfering with the normal function of receptors for angiogenic mediators should potently disrupt the angiogenesis cascade. To evaluate this concept, Millauer et al. The mutant lacks the intracellular domain but retains the extracellular and the transmembrane domain; as such, the mutant receptor remains cell associated but dysfunctional. By infecting endothelial cells with the retrovirus vector coding for the mutant receptor, heterodimerization occurs between the mutant receptor and the full-length, native Flk-1 receptor on endothelial cell membrane. Unlike the native homodimeric Flk-1 receptor, the heterodimer was unable to bring about signal transduction and endothelial cell activation. As a proof of principle, a combined ex vivo and in vivo strategy was used, where an ecotropic packaging cell line producing a retrovirus vector coding for the mutant receptor was co-implanted with glioblastoma cells in nude mice; the result was suppression of tumor growth compared with findings in control animals It is interesting that intratumoral administration of the retroviral supernatant also suppressed growth of primary glioblastomas. To accomplish this, an adenovirus vector Adsflt was designed to deliver a cDNA coding for a truncated form of the Flt-1 VEGF receptor that lacked the intracellular domain, the transmembrane domain, and part of the extracellular domain. The product expressed by the vector sFlt is a diffusible, soluble receptor molecule that can bind to VEGF molecules with high affinity. Irrespective of the responsible mechanism, when the Adsflt vector was administered in vivo to mice bearing primary or metastatic tumors that arose from syngeneic colon carcinoma cells, substantial tumor suppression was observed, and the treated animals had a statistically significant survival advantage. Importantly, the therapeutic effect was found to be regional, i. Since angiogenesis is the net result of a dynamic balance between the proangiogenic and antiangiogenic factors in the extracellular microenvironment of the tumor, increasing the local concentrations of endogenous inhibitors of angiogenesis should shift the balance between angiogenesis and antiangiogenesis in favor of the latter. To evaluate this concept, Tanaka et al. Importantly, the growth of gliomas in nude mice could be suppressed following intratumoral administration of the vectors; the treated tumors appeared hypovascular, and the treated animals had a survival advantage over the control animals In another study evaluating the concept of increasing the local concentration of inhibitors of angiogenesis 59 , the TSP-1 cDNA was transfected into breast carcinoma cells, followed by their injection into the mammary fat pads of nude mice. The resulting tumors were found to be smaller and to have a lower metastatic potential than the naive i. Although the gene therapy approach to antiangiogenesis therapy for solid tumors is still in its infancy, the preliminary data developed to date suggest gene therapy should live up to its theoretical potential of providing high, local concentrations of the therapeutic molecule, while avoiding the potential toxicity of systemic administration. However, there are several challenges that will have to be overcome before antiangiogenesis gene therapy becomes a useful strategy to treat human tumors. Some of these challenges are specific for gene therapy per se ; others are generic challenges for antiangiogenesis. It is apparent from experimental animal studies and from the early clinical studies of systemic antiangiogenesis therapy that, for antiangiogenesis therapy to be effective in treating tumors, the antiangiogenesis effects must be maintained for a long time 20 , If we assume that remaining tumor cells have the potential to express proangiogenic mediators, interruption of antiangiogenesis therapy has the potential risk of tipping the angiogenesis balance in favor of proangiogenesis, allowing the tumor to emerge from its dormancy. A successful gene therapy for antiangiogenesis should therefore have a sustained effect. This is an important challenge for current gene transfer vectors, which either inherently provide only transient expression e. There are various solutions to the challenge of maintaining persistent expression of a transgene following gene transfer. They include the following: 1 designing vectors to be more efficient in entering the target cell and transferring genes to the nucleus, 2 permanently incorporating the transgene into the target cell genome, 3 designing the vector to be stealthy with regard to detection by the hosts' innate and adaptive immune systems, 4 using pharmacologic agents to suppress host responses to the vectors, 5 designing the transgene to code for an antiangiogenic protein that has a longer biologic half-life in the target organ, and 6 using promoters that are resistant to shutdown by the host cell. Although there are risks to this approach in that the other antitumor gene strategy may eliminate the cells expressing the antiangiogenesis genes, the initial tumor reduction brought about by an antiangiogenesis treatment might be maintained by chronic tumor suppression from antitumor genes that function to suppress tumor growth by other mechanisms. In this context, gene therapy-based immunotherapy may be the best choice for combined therapy, in that it would provide tumorspecific suppression, while the gene therapy-based antiangiogenesis therapy would attack the tumor by a very different mechanism, independent of the cell site of the antiangiogenesis genes. While the local production of a therapeutic antiangiogenesis protein is an inherent advantage of gene therapy in that it limits the risk of promiscuous systemic antiangiogenesis, such a strategy suffers from an inability to treat widespread metastases. Thus, one challenge of future antiangiogenesis gene therapy is to target the vector or its transgene product to tumor-associated vessels, permitting systemic treatment of disseminated tumors. Such a goal may be achieved when more is known about the phenotypic differences between normal vessels and those that are induced by and support growing tumors. In this regard, there are emerging data suggesting that tumor neovasculature behaves differently from its normal counterpart in that, although the tumor neovasculature is composed of normal cells, its architecture is abnormal. For example, tumor blood vessels are leaky and aberrantly arranged, with unusual fan and spiral motifs, forming right angles and arteriovenous shunts 15 , Up-regulation of α v β 3 integrins is also a feature of new blood vessels in tumors, a biologic process believed to be critical for the survival and differentiation of vascular cells undergoing angiogenesis Since adenovirus vectors use α v β 3 integrins as an internalization signal, it may be possible to capitalize on this feature to design adenovirus vectors specific for active angiogenesis, such as occurs in growing tumors — Another possible target to achieve tumor vessel specificity is E-selectin One of the challenges to success of antiangiogenesis gene therapy is to ensure that the strategy will be applicable to a broad range of tumor types, regardless of their profiles of angiogenic mediators. A successful strategy will have to counteract the proangiogenic phenotype induced by VEGF, bFGF, and likely other angiogenic mediators. It may therefore be more rational to target genes that express products that function to interrupt processes downstream in the angiogenesis cascade. In this regard, gene therapy approaches that target the common signaling cascades in the end organ of angiogenesis, i. With a better appreciation of the critical and universal dependence of tumor progression on neovascularization, it is rational to hypothesize that suppression of this rate-limiting step could suppress the growth of a wide range of tumor types. As the cellular and molecular events that underlie tumor angiogenesis become better defined, rational strategies can be derived to apply this molecular tourniquet. An ideal antiangiogenesis strategy should be targeted to only the organs that contain the tumors and should not interfere with normal angiogenesis; it must achieve a high ratio of regional-to-systemic concentrations to minimize systemic toxicity; it must have a biologic half-life sufficient to counter the proangiogenesis phenotype of the tumor; and its antiangiogenesis effects should be regulatable. Gene transfer strategies potentially satisfy many of these requirements. Critical to the success of antiangiogenesis gene transfer is the fact that endothelial cells are activated or suppressed in trans , depending on the composition of the extracellular milieu. In this context, gene therapy for angiogenesis does not have to transduce all or any specific populations of cells in the target organs to achieve a high, local concentration of the antiangiogenesis proteins. Antiangiogenesis treatment is likely to be most effective in a low tumor burden state. In such a setting, therapeutic antiangiogenesis can be expected to prolong the state of tumor dormancy by suppressing micrometastases that remain despite successful treatment of the primary tumors. One appropriate clinical approach to using antiangiogenesis therapy in cancer is to combine it with conventional therapy to reduce the initial tumor burden, followed by its use in an adjuvant setting to prolong disease-free survival. Google Scholar. Google Preview. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Navbar Search Filter JNCI: Journal of the National Cancer Institute This issue JNCI Portfolio Medicine and Health Books Journals Oxford Academic Mobile Enter search term Search. Issues More Content Advance Articles Supplements Submit Author Guidelines Submission Site Why Publish with Us? Open Access Author Resource Centre Purchase Alerts About About JNCI Editorial Board Diversity, Equity, and Inclusion Advertising and Corporate Services Journals Career Network Policies Self-Archiving Policy Dispatch Dates Journals on Oxford Academic Books on Oxford Academic. JNCI Portfolio. Open Access Author Resource Centre Purchase Alerts About About JNCI Editorial Board Diversity, Equity, and Inclusion Advertising and Corporate Services Journals Career Network Policies Self-Archiving Policy Dispatch Dates Close Navbar Search Filter JNCI: Journal of the National Cancer Institute This issue JNCI Portfolio Medicine and Health Books Journals Oxford Academic Enter search term Search. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Why Use Gene Therapy to Deliver Antiangiogenesis Agents? Biology of Tumor Angiogenesis Relevant to Antiangiogenesis Gene Therapy. Antiangiogenesis With the Use of Gene Therapy. Challenges to Successful Antiangiogenesis Gene Therapy. Journal Article. Gene Therapy Strategies for Tumor Antiangiogenesis. Hwai-Loong Kong , Hwai-Loong Kong. Oxford Academic. Ronald G. Revision received:. Split View Views. Cite Cite Hwai-Loong Kong, Ronald G. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Close Navbar Search Filter JNCI: Journal of the National Cancer Institute This issue JNCI Portfolio Medicine and Health Books Journals Oxford Academic Enter search term Search. Abstract Based on the concept that solid tumors cannot grow without the generation of new blood vessels, there is growing interest in the use of antiangiogenesis agents to inhibit tumor growth. Open in new tab Download slide. Google Scholar Crossref. Search ADS. Google Scholar PubMed. OpenURL Placeholder Text. Review article: angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Google Scholar Google Preview OpenURL Placeholder Text. Tumor behavior in isolated perfused organs: in vitro growth and metastases of biopsy material in rabbit thyroid and canine intestinal segment. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. The implications of angiogenesis for the biology and therapy of cancer metastasis. Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. Tumor-associated angiogenesis: mechanisms, clinical implications, and therapeutic strategies. Anticancer strategies involving the vasculature: vascular targeting and the inhibition of angiogenesis. Controlling the vasculature: angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Dioleoylmelittin as a novel serum-insensitive reagent for efficient transfection of mammalian cells. Effect of size and serum proteins on transfection efficiency of poly 2-dimethylamino ethyl methacrylate -plasmid nanoparticles. Gene gun delivery of mRNA in situ results in efficient transgene expression and genetic immunization. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Cytotoxic T lymphocyte responses to proteins encoded by heterologous transgenes transferred in vivo by adenoviral vectors. Long-term gene expression and phenotypic correction using adenoassociated virus vectors in the mammalian brain. Adeno-associated virus AAV as a vector for gene transfer into glial cells of the human central nervous system. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Gene therapy against an experimental glioma using adeno-associated virus vectors. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Inhibition of angiogenesis and tumor growth following adenovirus vectormediated in vivo transfer of the human soluble Flt receptor gene. Google Scholar OpenURL Placeholder Text. Also, as a new targeted strategic approach, nanotechnology medical applications have been intensively studied to deliver anti-angiogenic drugs into the tumoral specific sites using nanomaterials as cerium oxide, gold, silver, copper, silica, based on carbon or hyaluronic acid and others Mukherjee and Patra, Besides their role in normal tissue maintenance, angiogenesis initiation may indicate a shift from tumor latency to malignant active growth and recurrence of the disease. The precise functions of pro- and anti-angiogenic factors and the interactions between them in tumor angiogenesis are not fully understood and the important question is how anti-angiogenic medicine can be improved. However, the mechanisms of induction of vascularization and subsequent development from precancerous lesions to micrometastases achieved by angiogenic strategies for vessel recruitment are not yet fully elucidated in all pathological cases. Specific agents that can block tumor vascularization are required to inhibit angiogenesis and tumor growth. This review summarizes angiogenic factors involved in each step of vessel development to present an integrated overview of tumor vascularization models such as cooption, intussusception, sprouting angiogenesis, vasculogenic mimicry, and angioblasts which, depending on the context, can be helpful for targeted or combined anti-angiogenic therapies. Moreover, we present the epigenetic changes in cancer which in contrast with genetic changes, are potentially reversible, increasing the prospect that epigenetic therapy will be able to mediate tumor fate. In addition to more disease-specific biomarkers, an important issue remains optimization of the dose and frequency of delivery of anti-angiogenic drugs. Current efforts for biomarker discovery in cancer have primarily focused on multi-gene expression patterns, but complementary analysis of DNA methylation signatures may lead to diagnostic and prognostic improvement and better cancer therapy strategies. The major limitations of drug delivery systems remain the lack of specificity. However, drug-specific therapies that use a lower dose of epi-drugs could improve the effectiveness and tolerability of treatments. Another approach that might improve cancer therapy is the optimization of the dose and duration of release of anti-angiogenic drugs, with potential to alleviate colateral damage that conventional treatments that are toxic to both tumor and normal cells might produce. Future directions for these treatments may include combined drug delivery systems that might target several types of anti-angiogenic factors for synergistic or additive therapeutic effects, and might increase the efficacy and specificity along with reduction of side effects. VA and CD were involved in study conception. IS and CB were involved in study design. VA wrote the manuscript with support from IS, CB, and CD. All authors reviewed and approved the final version of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. We gratefully acknowledge the funding from the project Competitiveness Operational Programme COP A1. Adair, T. Chapter 1, Overview of Angiogenesis. Google Scholar. Aggarwal, B. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. Almokadem, S. Volociximab in cancer. doi: PubMed Abstract CrossRef Full Text Google Scholar. Alumkal, J. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. New Drugs 33, — Angara, K. A novel neovascularization mechanism driving anti-angiogenic therapy AAT resistance in glioblastoma. Arroyo, A. Extracellular matrix, inflammation, and the angiogenic response. Asahara, T. Isolation of putative progenitor endothelial cells for angiogenesis. Science , — Baeriswyl, V. The angiogenic switch in carcinogenesis. Cancer Biol. Banfi, A. Therapeutic angiogenesis due to balanced single-vector delivery of VEGF and PDGF-BB. FASEB J. Bartel, D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell , — CrossRef Full Text Google Scholar. Bazmara, H. The vital role of blood flow-induced proliferation and migration in capillary network formation in a multiscale model of angiogenesis. PLoS One e Bazou, D. Flow-induced HDAC1 phosphorylation and nuclear export in angiogenic sprouting. Ben Mousa, A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J. Benelli, R. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. Bergers, G. Modes of resistance to anti-angiogenic therapy. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. Bhome, R. A top-down view of the tumor microenvironment: structure, cells and signaling. Bonauer, A. MicroRNAa controls angiogenesis and functional recovery of ischemic tissues in mice. Bottsford-Miller, J. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. Bridges, E. Notch regulation of tumor angiogenesis. Future Oncol. Bueno, M. Personalising and targeting antiangiogenic resistance: a complex and multifactorial approach. Butler, L. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Buysschaert, I. Genetics, epigenetics and pharmaco- epi genomics in angiogenesis. Cao, D. Bevacizumab improves survival in metastatic colorectal cancer patients with primary tumor resection: a meta-analysis. Carmeliet, P. Molecular mechanisms and clinical applications of angiogenesis. Nature , — Celic, T. Cha, S. MicroRNAc suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis. Cancer Res. Chappell, J. Local guidance of emerging vessel sprouts requires soluble Flt Cell 17, — Chen, D. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Cancer Drug Targets 11, — Chen, R. Methyltransferase G9a promotes cervical cancer angiogenesis and decreases patient survival. Oncotarget 8, — Chen, Y. Regulation of angiogenesis through a microRNA miRa that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood , — Chen, Z. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. Cheng, H. Inhibition of lymphangiogenic factor VEGF-C expression and production by the histone deacetylase inhibitor suberoylanilide hydroxamic acid in breast cancer cells. Cheng, Z. miRa inhibits tumor metastasis and angiogenesis by targeting FAK pathway. Cimmino, A. miR and miR induce apoptosis by targeting BCL2. Darland, D. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Das, S. Angiogenesis in glioblastoma. Dashwood, R. Dietary histone deacetylase inhibitors: from cells to mice to man. De Smet, F. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. De Spiegelaere, W. Intussusceptive angiogenesis: a biologically relevant form of angiogenesis. del Toro, R. Identification and functional analysis of endothelial tip cell-enriched genes. Deroanne, C. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene 21, — Deryugina, E. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. Ding, S. MiR in cardiovascular diseases: physiology and pathology. Donnem, T. Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med. Duan, Y. DOT1L promotes angiogenesis through cooperative regulation of VEGFR2 with ETS Oncotarget 7, — Duffy, A. Vascular Endothelial Growth Factor VEGF and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. Madame Curie Bioscience Database. Austin, TX: Landes Bioscience. Dulloo, I. Hypoxia-inducible TAp73 supports tumorigenesis by regulating the angiogenic transcriptome. Cell Biol. Eguchi, J. Gene expression and immunohistochemical localization of basic fibroblast growth factor in renal cell carcinoma. Erber, R. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. Ergün, S. Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis 4, — Esser, J. The neuronal transcription factor NPAS4 is a strong inducer of sprouting angiogenesis and tip cell formation. Fan, L. The hypoxia-inducible factor pathway, prolyl hydroxylase domain protein inhibitors, and their roles in bone repair and regeneration. Fan, Y. MiRc inhibits glioma cell proliferation, migration, invasion and angiogenesis. Fan, Z. MicroRNA promotes angiogenesis in acute myocardial infarction. Fasanaro, P. MicroRNA modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand ephrin-A3. Ferrara, N. Vascular endothelial growth factor: basic science and clinical progress. Clinical applications of angiogenic growth factors and their inhibitors. Ferrari, G. TGF-β1 induces endothelial cell apoptosis by shifting VEGF activation of p38 MAPK from the prosurvival p38β to proapoptotic p38α. Fish, J. miR regulates angiogenic signaling and vascular integrity. Cell 15, — Fitzgerald, G. The warburg effect in endothelial cells and its potential as an anti-angiogenic target in cancer. Cell Dev. Folberg, R. Vasculogenic mimicry and tumor angiogenesis. Fox, J. Targeting of TGFβ signature and its essential component CTGF by miR correlates with improved survival in glioblastoma. RNA 19, — Fraineau, S. Epigenetic activation of pro-angiogenic signaling pathways in human endothelial progenitors increases vasculogenesis. Stem Cell Rep. Gaengel, K. Endothelial-mural cell signaling in vascular development and angiogenesis. Gavard, J. VE-cadherin and claudin it takes two to tango. Ge, H. Overview of advances in vasculogenic mimicry — a potential target for tumor therapy. Cancer Manag. Geng, H. HDAC4 protein regulates HIF1α protein lysine acetylation and cancer cell response to hypoxia. Geng, L. TGF-beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS One 8:e Gerhardt, H. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. Gerwins, P. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Ghosh, A. MiRNAa-3p suppresses tumor growth, migration, invasion and angiogenesis in hepatocellular carcinoma by targeting VEGFA, VEGFR1, VEGFR2, HGF and MMP2. Cell Death Dis. Gianni-Barrera, R. To sprout or to split? VEGF, Notch and vascular morphogenesis. Goel, S. Normalization of the vasculature for treatment of cancer and other diseases. Gong, W. Expression and clinical significance of methyl-CpG binding domain protein 2 in high-grade serous ovarian cancer. Groppa, E. EMBO Rep. Guarani, V. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Hamer, H. Review article: the role of butyrate on colonic function. Aliment Pharmacol. Hammond, E. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. R Coll. Hao, H. Matrix metalloproteinase-9 MMP-9 as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors Hassan, F. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Heissig, B. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Hellebrekers, D. Dual targeting of epigenetic therapy in cancer. Acta , 76— Angiostatic activity of DNA methyltransferase inhibitors. Cancer Ther. Hellström, M. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Hilberg, F. Triple angiokinase inhibitor nintedanib directly inhibits tumor cell growth and induces tumor shrinkage via blocking oncogenic receptor tyrosine kinases an external file that holds a picture, illustration, etc. Hillen, F. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. Holash, J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Huang, H. Matrix metalloproteinase-9 MMP-9 as a cancer biomarker and mmp-9 biosensors: recent advances. Huang, Z. Roles of main pro- and anti-angiogenic factors in tumor angiogenesis. World J. Humphries, J. Integrin ligands at a glance. Cell Sci. Iizuka, N. Anti-angiogenic effects of valproic acid in a mouse model of oxygen-induced retinopathy. Jain, R. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Jiang, T. CD is a coreceptor for VEGFR-2 in tumor angiogenesis. Jones, B. The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet. Kalka, C. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. A 97, — Kang, F. Effects of trichostatin A on HIF-1α and VEGF expression in human tongue squamous cell carcinoma cells in vitro. Kazemi, S. Differential role of bFGF and VEGF for vasculogenesis. Cell Physiol. Kim, M. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Knies-Bamforth, U. c-Myc interacts with hypoxia to induce angiogenesis in vivo by a vascular endothelial growth factor-dependent mechanism. Krock, B. Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2, — Krstic, M. Isoform-specific promotion of breast cancer tumorigenicity by TBX3 involves induction of angiogenesis. Lab Invest. Kuczynski, E. Vessel co-option in cancer. Kuehbacher, A. Role of dicer and drosha for endothelial microRNA expression and angiogenesis. Küsters, B. Vascular endothelial growth factor-A induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Larrivée, B. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. Lee, D. MicroRNA promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. A , — Lee, J. Hypoxia-inducible factor HIF-1 alpha: its protein stability and biological functions. Trichostatin A resistance is facilitated by HIF-1α acetylation in HeLa human cervical cancer cells under normoxic conditions. Oncotarget 9, — Inhibition of HDAC3- and HDAC6-promoted survivin expression plays an important role in saha-induced autophagy and viability reduction in breast cancer cells. LSD1 demethylates HIF1α to inhibit hydroxylation and ubiquitin-mediated degradation in tumor angiogenesis. Oncogene 36, — Lee, S. Maintenance of vascular integrity in the embryo requires signaling through the fibroblast growth factor receptor. Lee, Y. Molecular mechanism of SAHA on regulation of autophagic cell death in tamoxifen-resistant MCF-7 breast cancer cells. Lezcano, C. Merkel cell carcinoma expresses vasculogenic mimicry: demonstration in patients and experimental manipulation in xenografts. Li, Y. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. Clin Invest ; : — Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol ; : — Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A ; 97 : — St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science ; : — Liau G, Su EJ, Dixon KD. Clinical efforts to modulate new blood vessel formation in the adult; a comparison of gene therapy versus conventional approaches. Drug Discovery Today ; 6 : 19 — Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A ; 98 : — Jia S, Shu F, Li H, Fushu H, Ziu R. Clin Hemorheology Microcircul ; 23 : —7. Chen CT, Lin J, Li Q, Phipps SS, Jakubczak JL, Stewart DA, et al. Antiangiogenic gene therapy for cancer via systemic administration of adenoviral vectors expressing secretable endostatin. Hum Gene Ther ; 11 : — Ding I, Sun JZ, Fenton B, Liu WM, Kimsely P, Okunieff P, et al. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res ; 61 : — Szary J, Szala S. Intra-tumoral administration of naked plasmid DNA encoding mouse endostatin inhibits renal carcinoma growth. Int J Cancer ; 91 : —9. Regulier E, Pau S, Marigliano M, Kintz J, Poitevin Y, Ledoux C, et al. Adenovirus-mediated delivery of antiangiogenic genes as an antitumor approach. Cancer Gene Ther ; 8 : 45 — Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci U S A ; 97 : —7. Feldman AL, Alexander HR, Hewitt SM, Lorang D, Thiruvathukal CE, Turner EM, et al. Effect of retroviral endostatin gene transfer on subcutaneous and intraperitoneal growth of murine tumors. J Natl Cancer Inst ; 93 : — Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo CR, et al. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci U S A ; 98 : —9. Kuo CJ, LaMontagne KR Jr, Garcia-Cardena G, Ackley BD, Kalman D, et al. J Cell Biol ; : — Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross R, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency SCID -X1 disease. Science ; : —9. Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis. Issues, problems, consensus: An expert panel summary. Circulation ; : E73 — Webster KA. Therapeutic angiogenesis: a case for targeted, regulated gene delivery. Crit Rev Eukaryot Gene Expr ; 10 : — Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Navbar Search Filter JNCI: Journal of the National Cancer Institute This issue JNCI Portfolio Medicine and Health Books Journals Oxford Academic Mobile Enter search term Search. Issues More Content Advance Articles Supplements Submit Author Guidelines Submission Site Why Publish with Us? Open Access Author Resource Centre Purchase Alerts About About JNCI Editorial Board Diversity, Equity, and Inclusion Advertising and Corporate Services Journals Career Network Policies Self-Archiving Policy Dispatch Dates Journals on Oxford Academic Books on Oxford Academic. JNCI Portfolio. Open Access Author Resource Centre Purchase Alerts About About JNCI Editorial Board Diversity, Equity, and Inclusion Advertising and Corporate Services Journals Career Network Policies Self-Archiving Policy Dispatch Dates Close Navbar Search Filter JNCI: Journal of the National Cancer Institute This issue JNCI Portfolio Medicine and Health Books Journals Oxford Academic Enter search term Search. Advanced Search. Search Menu. |
| Angiogenic and antiangiogenic gene therapy | The simplest non-viral gene delivery system uses naked expression vector DNA. Direct injection of free DNA into certain tissues, particularly muscle, has been shown to produce surprisingly high levels of gene expression, and the simplicity of this approach has led to its adoption in a number of clinical protocols. However, naked DNA and peptides have a very short half life due to in vivo enzymatic degradation. Plasmid DNA suffers from low transfection efficiency. However, there are reports showing efficacy of naked plasmid DNA administration. Intra-tumoral administration of naked plasmid DNA encoding mouse endostatin inhibits renal carcinoma growth [ ]. Intramuscular administration of the endostatin gene could significantly retard the growth of metastatic brain tumors [ ]. A single intramuscular administration of the endostatin gene could secret endostatin for up to 2 weeks and could inhibit systemic angiogenesis [ ]. Injected endostatin gene also inhibited both the growth of primary tumors and the development of metastatic lesions. Thus, these results demonstrate the potential utility of intramuscular delivery of an antiangiogenic gene for treatment of disseminated cancers. However, in comparison to naked DNA, complexes of plasmid DNA with liposomes are relatively more stable with higher potency for transfection [ ]. Liposomes are microscopic spherical vesicles of phospholipids and cholesterol. Recently, liposomes have been evaluated as delivery systems for drugs and have been loaded with a great variety of molecules such as small drug molecules, proteins, nucleotides and even plasmids. The advantages of using liposomes as drug carriers are that they can be injected intravenously and when they are modified with lipids which render their surface more hydrophilic, their circulation time in the bloodstream can be increased significantly. They can be targeted to the tumor cells by conjugating them to specific molecules like antibodies, proteins, and small peptides [ ]. The cationic liposomes can significantly improve systemic delivery and gene expression of DNA [ ]. Systemic, liposome-mediated administration of angiostatin could suppress the growth of melanoma tumors in mice [ ]. Similar findings were observed by Chen and co-workers with angiostatin and endostatin [ ]. It has been shown that angiogenic ECs take fold more cationic liposome:DNA complexes than corresponding normal ECs. Thus, preferential uptake of cationic liposomes could be used to target diagnostic or therapeutic agents selectively to angiogenic blood vessels in tumors [ ]. Liposomes modified with angiogenic homing peptide for ECs can strongly suppress tumor growth compared to unmodified liposomes [ ]. Janssen et al showed that the coupling of cyclic RGD-peptides to the surface of PEG-liposomes can target to tumor endothelium [ ]. Tumor vessel-targeted liposomes can also be used to efficiently deliver therapeutic doses of chemotherapy [ ]. Antisense oligodeoxynucleotides ODNs are synthetic molecules that block mRNA translation. They can be used as a tool to inhibit mRNA translation of a diseased gene. There are reports demonstrating use of VEGF and VEGFR antisense RNA in preclinical models. Angiogenesis and tumorigenicity as measured by MVD and tumor volume, respectively of human esophageal squamous cell carcinoma can be effectively inhibited by VEGF antisense RNA [ ]. Im and co-workers used an adenovirus to transfer antisense VEGF sequence into glioma cells in vitro and in vivo [ ]. The treatment resulted in reduction of the level of the endogenous VEGF mRNA and protein and inhibited growth of glioma tumors. VEGF mediated neovascularization can also be inhibited by combination of antisense oligonucleotides to VEGFR1 and VEGFR2 [ ]. Expression of antisense RNA to Ang1 could reduce tumor volume, decrease MVD and increase apoptosis in nude mice [ ]. Expression of antisense to integrin subunit beta 3 inhibits microvascular EC capillary tube formation in fibrin, with the extent of down-regulation correlating with the extent of tube formation inhibition [ ]. The ability of small dsRNA to suppress the expression of a gene corresponding to its own sequence is called RNA interference RNAi. The discovery of RNAi has added a promising tool to the field of molecular biology. Introducing the SiRNA corresponding to a particular gene will knock out the cell's own expression of that gene. The application of SiRNA to silence gene expression has profound implications for the intervention of human diseases including cancer. There are published reports using SiRNA to silence expression of angiogenic genes. SiRNA targeted to either subunit of the alpha6beta4 a laminin adhesion receptor integrin reduced its cell surface expression and resulted in decreased invasion of MDA-MB breast carcinoma cells [ ]. The disadvantage to simply introducing dsRNA fragments into a cell is that gene expression is only temporarily reduced. However, Brummelkamp et. developed a new vector system, named pSUPER, which directs the synthesis of siRNA in mammalian cells [ ]. The authors have shown that siRNA expression mediated by this vector causes persistent and specific down-regulation of gene expression, resulting in functional inactivation of the targeted gene over longer periods of time. VEGF carries out multifaceted functions in tumor development. DNA-vector based RNAi, in which RNAi sequences targeting VEGF isoforms, has potential applications in isoform-specific knock-down of VEGF [ ]. However, the disadvantages of non viral vectors — stability, non-specific uptake by various tissues, poor adsorption, short half life in the circulation, aggregate formation, and low in-vivo potency for cell transfection — continue to limit its use. The main characteristics of any viral vector are easy purification into high titers, to mediate targeted gene delivery and prolonged gene expression with minimal side effects. There are 5 main classes of clinically applicable viral vectors; adenoviruses, adeno-associated viruses AAVs , retroviruses, lentiviruses, and herpes simplex-1 viruses HSV-1s [ ]. The main difference between these vectors is retroviruses and lentiviruses can integrate into the genome, whereas the other three classes predominantly persist as extrachromosomal episomes. Although the advantage of chromosomal integration is long term transgene expression, these vectors can infect dividing cells only. The non-integrated viral vectors can infect non-dividing cells, but are not a favorable choice to bring about stable genetic change. Adenovirus is a double stranded DNA virus. It binds initially to the target cell through the viral fiber protein; however, the subsequent cell entry involves interaction between the viral capsid penton proteins and integrins on the target cell. Adenoviruses can be produced in high titers and can efficiently deliver the therapeutic gene. Replication-defective adenoviral vectors are indeed particularly well suited for cancer gene therapy as they lead to a transient, but robust, expression of the transgene, and efficient in vivo gene transfer has been reported especially in the liver after systemic injection. However, they do not integrate into the host genome and the gene expression is transient. Also, adenovirus elicits an immune response resulting in an elimination of vector expressing cells. Despite these apparent drawbacks, the adenovirus remains a popular vector for gene therapy due to its high gene transfer efficiency and high level of expression in a wide variety of cell types. Adenovirus can be targeted to pulmonary endothelium by complexing it with a bispecific antibody to viral particle and angiotensin-converting enzyme [ ]. Bilamellar cationic liposomes can also be used to encapsulate adenovirus. The encapsulated adenovirus can transduce CAR negative cells and is resistant to the neutralizing anti-adenoviral antibodies, allowing the readministration of the adenovirus [ ]. Retroviruses are a class of enveloped viruses containing a single stranded RNA molecule as the genome. Retrovirus vectors have been used in the majority of human gene transfers. They are able to efficiently integrate permanently into the human genome where they provide the basis for permanent expression of up to 8—9 kb of foreign DNA. Simple retroviruses, such as murine leukemia virus MLV , and the vectors derived from them, require cell division for infection and thus possess a degree of inherent specificity for the rapidly dividing cells of neoplastic tissue [ ]. Though transgene expression is usually adequate in vitro, prolonged expression is difficult to attain in vivo. Also, retroviruses are inactivated by complement proteins and inflammatory IFN, specifically IFN-alpha and IFN-gamma [ , ]. A major shortcoming of retrovirus-derived vectors is their tendency to revert to replication-competent retrovirus RCR , which could lead to fatal neoplasms. With the use of the latest packaging cell lines and vectors, the risk of RCR-generation has been drastically reduced. Currently, the greatest safety concern of using retroviral vectors is related to the risk of malignant transformation following oncogene activation due to random retroviral genomic integration and will be discussed in more detail below. The adeno-associated virus AAV is a vector that combines some of the advantages of both the adenoviral and retroviral vectors. It can efficiently transfer genes to a number of different cell types. The broad host range, low level of immune response, and longevity of gene expression observed with these vectors has enabled the initiation of a number of clinical trials using this gene delivery system [ ]. A potential barrier, however, is the low transduction efficiencies of recombinant AAV vectors. AAV tropism can be genetically engineered by use of phage display-derived peptides to generate vectors that are selective for the vasculature [ ]. The journal Nature recently reported concerns that there is a possibility that recombinant AAV vectors may cause or contribute to cancer in gene therapy subjects [ ]. However, because of infrequent integration efficiency of AAV, the risk of cancer in current AAV trials is negligible [ ]. Lentiviral vectors represent a new vector system that can achieve permanent integration of the gene into non-dividing cells. Gene transfer can be achieved in very quiescent cells, nondividing or terminally differentiated cells such as neurons. Lentiviral vectors are especially useful in transducing cells which lack receptors for adenoviruses. A broad tissue tropism for lentivirus can be achieved using variety of viral envelopes [ ]. So far, lentiviral vectors expressing matrix metalloproteinase-2 MMP-2 , angiostatin and endostatin have been developed [ , ]. However, lentivirus has a low transduction efficiency for ECs and may result in significant vector-associated cytotoxicity [ ]. The herpes simplex virus HSV thymidine kinase gene tk therapy with ganciclovir forms the basis of a widely used strategy for suicide gene therapy [ ]. HSV can also be used to deliver a therapeutic gene of interest, especially to the nervous system. The advantages in using HSV include its wide host range, its ability to accommodate large genes, and its ability to establish long-lived asymptomatic infections in neuronal cells. However, the virus's ability to replicate lytically in the brain, under some circumstances causing encephalitis, has led to fears about its potential safety for ultimate use in humans. The systemic delivery of viral vectors can lead to non specific uptake by various different tissues and hence result into systemic toxicity because of transgene expression. Local delivery can circumvent this problem; however, many times the tumor is not accessible for local injection. Hence, transgene expression in the targeted tissue is very important. Targeting tumor vasculature by gene therapy represents an ideal target, as tumor blood vessels are easily accessible to systemically applied vectors. Certain considerations such as vascular dependency of a tumor, the differences between tumor associated and normal vasculature, and accessibility of tumor vasculature to circulating vectors must be addressed. Targeted viral vectors can be constructed in several ways. The first approach is pseudotyping, in which one species of virus is made to incorporate the envelope protein of another virus. Adeno-associated virus genome with its inverted terminal repeats can be packaged in the capsids of different serotypes which enables transduction with broad specificity [ ]. The second approach is to genetically modify the viral capsid protein to incorporate a small peptide coding for a specific receptor and hence, allow targeting by ligand-receptor internalization. Tissue specific targeting can also be achieved by conjugating specific antibodies to receptors onto the viral capsids [ ]. Various transductional and transcriptional approaches have also been devised to improve targeting of adenoviral vectors. Using this approach, Reynolds et al increased the selectivity of transgene expression by , for lung versus liver, the usual site of vector sequestration [ ]. Another strategy is to use vectors guided by EC specific promoters, such as the promoters for endoglin, endothelin-1 gene, and von Willebrand factor. The murine preproendothelin-1 promoter is highly specific for ECs and could be use to achieve very high levels of gene expression in vascularized tumor metastases compared to normal or less vascularized primary tumor [ ]. Whereas most antiangiogenic agents prevent new blood vessel formation, vascular targeting agents VTAs destroy pre-existing blood vessels of solid tumors. VTAs can be more active in large tumors and produce a characteristic pattern of widespread central necrosis. Combination of VTAs and angiogenic inhibitors can lead to synergistic effects. Two major types of VTAs are ligand-directed VTAs to deliver gene product to tumor endothelium and small molecule VTAs that exploit pathophysiological differences between tumor and normal endothelium [ ]. The ligand-directed VTAs use ligands specifically expressed on the tumor endothelium, eg. VEGF receptors, αvβ3 integrins, tissue factor, and cell adhesion molecules like VCAM A small molecule VTA described thus far is the tubulin binding agent combretastatin, which destabilizes the tubulin cytoskeleton. It has antiproliferative and cytotoxic effects on both proliferating tumor and ECs. It results in extensive and prolonged shut-down of blood flow in established tumor blood vessels, with much less effect in normal tissues [ ]. Recently, bacteriophage vectors have been developed for targeted gene delivery of antiangiogenic VTAs. Phage display represents a novel method of individually displaying up to tens of billions of peptides, proteins, including human antibodies and enzymes, on the surface of a small bacterial virus called a phage. Phage display allows producing and searching through large libraries of peptides and proteins to rapidly identify those compounds that bind with high affinity and high specificity to targets of interest. Pasqualini and Ruoslahti first distinguished active proliferating microvascular ECs and quiescent nonproliferating ECs using an in vivo phage display technique [ ]. The phage display peptide libraries have been used to identify peptides that home to tumors through the circulation and that specifically bind to the tumor ECs or lymphatic cells [ , ]. Phage displaying an Arg-Gly-Asp RGD -containing peptide binds to alpha v integrins with high affinity and homes to tumors when injected intravenously into tumor-bearing mice. Phage displaying the cyclic peptide His-Try-Gly-Phe HWGF specifically targets angiogenic blood vessels in vivo and specifically inhibits MMP-2 and MMP-9 metalloproteinases [ ]. MMP-2 can directly bind to αvβ3 integrin on tumor cells [ ]. These tumor endothelial specific signatures were later described by Dr. Folkman as an angiogenic zip codes [ ]. The picture of integrins and their ligands is very complex. The αvβ3 integrin is expressed on proliferating ECs such as those present in growing tumors of various origins. VEGF-induced EC migration requires interaction between VEGF receptor2 VEGFR2 and αvβ3 to drive the activation of downstream mitogenic pathways [ ]. Several studies have demonstrated that αvβ3 is involved in melanoma cell invasion and promotes metastasis reviewed in [ ]. Although the precise mechanisms of αvβ3-promoted tumor progression are not clear, various studies suggest roles for αvβ3 in selective tumor cell migration, generation of growth and survival signals, intracellular signaling and generation of MMPs [ ]. It has been observed that antibody to αvβ3 integrin can block angiogenesis without affecting the normal vasculature [ , ]. The αvβ3 integrin can also be blocked with small peptides containing Arg-Gly-Asp RGD amino acid sequence. Exposure of human EC to TNF and interferon-γ decreases αvβ3-dependent EC adhesion and survival [ ]. Other integrins such as αvβ5, and αvβ1 are also considered very significant for the regulation of angiogenesis. The β3 chain play a significant role in promoting angiogenesis, although the lack of it can also promote angiogenesis, given that the angiogenesis inhibitor tumstatin binds through this receptor [ 41 ]. Also, the expression of integrin αvβ6, a fibronectin receptor, promotes migration and invasion in squamous carcinoma cells [ ]. Bacteriophages expressing ligands for specific receptors like integrins, growth factors, and antibody can be made to infect mammalian cells [ — ]. The phage vectors are an attractive alternative to existing animal viral vectors because they lack intrinsic tropism for mammalian cells and can be produced in bacteria in large titers. Burg et al tested 14 human cancer cell lines from different tissues, showing viral transduction efficiency varies from 0. However the transduction efficiency can be improved substancially by treating cells with camptothecin [ ]. Thus, using specific targets in the tumor endothelium, it is possible to target gene therapy specifically to the tumor endothelium. In recent years, significant effort has been devoted to developing nanotechnology for drug delivery since it offers a suitable means of delivering small molecular weight drugs as well as macromolecules such as proteins, peptides or genes [ ]. Nanoparticles NPs are submicron-sized polymeric colloidal particles with a therapeutic agent of interest encapsulated within their polymeric matrix or adsorbed or conjugated onto the surface [ ]. NPs have in general relatively higher intracellular uptake because of their smaller size. The therapeutic efficacy of the NPs could be due to their ability to protect the therapeutic agent from degradation due to lysosomal enzymes and sustained intracellular retention. Nanoparticles conjugated to the contrast agent like gadolinium can be used in magnetic resonance based imaging techniques [ , ]. They can be targeted to tumor endothelium by covalently coupling to different ligands or antibodies [ ]. David Cheresh's group at the Scripps Research Institute showed that NPs coupled to an integrin αvβ3-targeting ligand can deliver genes selectively to angiogenic blood vessels in tumor-bearing mice with no detectable levels in the other tissues [ ]. Further, the authors showed that NPs conjugated to a mutant Raf gene blocks endothelial signaling and angiogenesis in response to multiple growth factors. Despite the tantalizing promise of gene therapy, there has been much recent alarm in the field and no review of the current state of and potential future role of gene therapy would be complete without noting the major current controversy in the field. The first true successes of gene therapy in clinical trials were reported in and , in which scientists successfully treated children suffering from SCIDs by retrovirally transducing hematopoietic stem cells with the gamma-c gene [ 32 ]. Unfortunately, 2 of the 10 children subsequently developed leukemia-like conditions, eventually attributed to retroviral vector integration in proximity to the LMO2 proto-oncogene promoter, leading to aberrant transcription and expression of LMO2 [ ]. These cases garnered enormous attention from scientists, regulators and the general public [ ]. Reaction varied but some countries even imposed a general moratorium on trials involving retroviral gene transfer. Though in most countries trials have now been allowed to resume, the reaction has thrown the field of gene therapy into recession and has discouraged many scientists from starting new clinical trials [ ]. As addressed above, the development of better vectors remains the cornerstone for advancing the field of antiangiogenic gene therapy of cancer. Important concerns about oncogene activation by retroviral integrating vector systems remain. Also, currently available nonviral vectors are not very efficient. Studies have shown advantages to combining antiangiogenic agents with each other, as well as with chemotherapy and radiation [ ]. Combining agents that target a number of different and synergistic pathways may yield improved antitumor effects and long term suppression of metastatic progression. For example, the combination of SU, VEGFR2 tyrosine kinase inhibitor and low-dose endostatin reduced tumor growth more efficiently than monotherapy alone [ ]. Whereas SU specifically inhibits vascular endothelial growth factor signaling, low-dose endostatin is able to inhibit a broader spectrum of diverse angiogenic pathways directly in the endothelium. Gene therapy strategies certainly need to be further evaluated in these combination settings. More specific to the field of antiangiogenic gene therapy, there is much enthusiasm for the role that antiangiogenic agents may play in preventive therapy. Because antiangiogenic therapy is generally less toxic and less susceptible to acquired resistance, angiogenesis inhibitors have been considered an ideal prophylactic agent in patients at high risk for cancer or for recurrence of cancer [ 29 ]. At our institution, we are currently recruiting patients with previously resected recurrent or metastatic colorectal cancer and randomizing these patients to receive thalidomide or placebo. Gene therapy offers an ideal strategy for long term, continuous production of antiangiogenic agents that may prevent development of primary or recurrent disease. Additionally, many leaders in the field of angiogenesis now believe that some of the most important future cancer therapies may not completely eradicate all tumor cells in an individual but, instead, may turn cancer into a chronic manageable disease [ 32 ]. Gene therapy strategies leading to increased production of endogenous angiogenesis inhibitors would seem perfectly suited to support such an approach by tipping the balance toward a more antiangiogenic state. Enthusiasm for antiangiogenic approaches to cancer therapy has never been higher, given the recent approval by the FDA for the first angiogenesis inhibitor and given the recent encouraging clinical trial data [ ]. The versatility shown by the field of antiangiogenesis has enabled many human cancers to be realistically considered for therapeutic intervention and has considerably broadened the scope of gene therapy. Because of the difficulties and high costs of manufacturing numerous endogenous inhibitors of angiogenesis and because of the need for chronic administration of these agents, gene therapy remains an exciting strategy to circumvent these difficulties. Although no clinical gene therapy trials to date have utilized a focused antiangiogenic strategy, an abundance of preclinical research demonstrates that gene therapy-based antiangiogenesis approaches are an effective means of controlling and even eradicating tumor growth in animal models. As better vectors are developed, combination strategies continue to evolve, and increased understanding of the complex role that endogenous angiogenesis inhibitors play in tumor growth and progression takes place, antiangiogenic gene therapy will certainly be evaluated in future clinical trials. Antiangiogenic Gene Therapy: Recent Developments. The figure depicts different modes of gene therapy directed to tumor endothelial cell EC and its microenvironment. The expression of EC specific cell surface molecules like, vascular endothelial growth factor receptor VEGFR , E selectin or angiogenic growth factors VEGF, FGF, PDGF produced by tumor cells can be inhibited by specific antibodies or antisense RNA or using gene specific SiRNA. The targeted gene therapy can be achieved by using either viral vectors or nanoparticles carrying ligand RGD to endothelial cell surface specific receptors αvβ5, αvβ3 to target antiangiogenic genes to tumor vasculature. The inhibitors can also selectively express in EC using endothelial cell specific promoters like endothelin 1 EDT1. Ad: adenovirus; AAV: adeno-associated virus; Retro: retrovirus; VEGF: vascular endothelial growth factor; FGF: fibroblast growth factor; PDGF: platelet derived growth factor; TNF: tumor necrosis factor; RGD: Arginine-glycine-aspartic acid; SiRNA: small interfering RNA. Folkman J: Tumor angiogenesis: therapeutic implications. N Engl J Med. CAS PubMed Google Scholar. Satchi-Fainaro R, Puder M, Davies JW, Tran HT, Sampson DA, Greene AK, Corfas G, Folkman J: Targeting angiogenesis with a conjugate of HPMA copolymer and TNP Nat Med. Ribatti D, Vacca A, Presta M: The discovery of angiogenic factors: a historical review. Gen Pharmacol. Folkman J, Klagsbrun M: Angiogenic factors. Dameron KM, Volpert OV, Tainsky MA, Bouck N: Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE: Halting angiogenesis suppresses carcinoma cell invasion. Volpert OV, Dameron KM, Bouck N: Sequential development of an angiogenic phenotype by human fibroblasts progressing to tumorigenicity. Bertolini F, Mancuso P, Gobbi A, Pruneri G: The thin red line: angiogenesis in normal and malignant hematopoiesis. Exp Hematol. Holmgren L, O'Reilly MS, Folkman J: Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Breakthrough of the year. The runners-up. Sedlacek HH: Pharmacological aspects of targeting cancer gene therapy to endothelial cells. Crit Rev Oncol Hematol. Vartanian RK, Weidner N: Correlation of intratumoral endothelial cell proliferation with microvessel density tumor angiogenesis and tumor cell proliferation in breast carcinoma. Am J Pathol. PubMed Central CAS PubMed Google Scholar. Boehm T, Folkman J, Browder T, O'Reilly MS: Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engstrom A, Timpl R, Welsh M, Claesson-Welsh L: Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D: Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R: Tumor response to radiotherapy regulated by endothelial cell apoptosis. Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J: Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. Folkman J: Angiogenesis and apoptosis. Semin Cancer Biol. Gasparini G: Clinical significance of determination of surrogate markers of angiogenesis in breast cancer. Munshi NC, Wilson C: Increased bone marrow microvessel density in newly diagnosed multiple myeloma carries a poor prognosis. Semin Oncol. Hlatky L, Hahnfeldt P, Folkman J: Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. PubMed Google Scholar. Mancuso P, Calleri A, Cassi C, Gobbi A, Capillo M, Pruneri G, Martinelli G, Bertolini F: Circulating endothelial cells as a novel marker of angiogenesis. Adv Exp Med Biol. Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL: Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE: Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Hendrix MJ, Seftor EA, Hess AR, Seftor RE: Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, Trent JM, Meltzer PS, Hendrix MJ: Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Thies A, Mangold U, Moll I, Schumacher U: PAS-positive loops and networks as a prognostic indicator in cutaneous malignant melanoma. J Pathol. Makitie T, Summanen P, Tarkkanen A, Kivela T: Microvascular loops and networks as prognostic indicators in choroidal and ciliary body melanomas. Kerbel R, Folkman J: Clinical translation of angiogenesis inhibitors. Kisker O, Becker CM, Prox D, Fannon M, D'Amato R, Flynn E, Fogler WE, Sim BK, Allred EN, Pirie-Shepherd SR, Folkman J: Continuous administration of endostatin by intraperitoneally implanted osmotic pump improves the efficacy and potency of therapy in a mouse xenograft tumor model. Bergers G, Benjamin LE: Tumorigenesis and the angiogenic switch. Folkman J, Kalluri R: Cancer without disease. Marx J: Angiogenesis. A boost for tumor starvation. Liau G, Su EJ, Dixon KD: Clinical efforts to modulate angiogenesis in the adult: gene therapy versus conventional approaches. Drug Discov Today. Volpert OV, Alani RM: Wiring the angiogenic switch: Ras, Myc, and Thrombospondin Cancer Cell. de Fraipont F, Nicholson AC, Feige JJ, Van Meir EG: Thrombospondins and tumor angiogenesis. Trends Mol Med. Vailhe B, Feige JJ: Thrombospondins as anti-angiogenic therapeutic agents. Curr Pharm Des. Jin RJ, Kwak C, Lee SG, Lee CH, Soo CG, Park MS, Lee E, Lee SE: The application of an anti-angiogenic gene thrombospondin-1 in the treatment of human prostate cancer xenografts. Cancer Gene Ther. Liu P, Wang Y, Li YH, Yang C, Zhou YL, Li B, Lu SH, Yang RC, Cai YL, Tobelem G, Caen J, Han ZC: Adenovirus-mediated gene therapy with an antiangiogenic fragment of thrombospondin-1 inhibits human leukemia xenograft growth in nude mice. Leuk Res. O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J: Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R: Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. J Biol Chem. Hanai J, Dhanabal M, Karumanchi SA, Albanese C, Waterman M, Chan B, Ramchandran R, Pestell R, Sukhatme VP: Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. Folkman J: Angiogenesis inhibitors: a new class of drugs. Cancer Biol Ther. Herbst RS, Hess KR, Tran HT, Tseng JE, Mullani NA, Charnsangavej C, Madden T, Davis DW, McConkey DJ, O'Reilly MS, Ellis LM, Pluda J, Hong WK, Abbruzzese JL: Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. Thomas JP, Arzoomanian RZ, Alberti D, Marnocha R, Lee F, Friedl A, Tutsch K, Dresen A, Geiger P, Pluda J, Fogler W, Schiller JH, Wilding G: Phase I pharmacokinetic and pharmacodynamic study of recombinant human endostatin in patients with advanced solid tumors. Feldman AL, Restifo NP, Alexander HR, Bartlett DL, Hwu P, Seth P, Libutti SK: Antiangiogenic gene therapy of cancer utilizing a recombinant adenovirus to elevate systemic endostatin levels in mice. Feldman AL, Alexander HR, Hewitt SM, Lorang D, Thiruvathukal CE, Turner EM, Libutti SK: Effect of retroviral endostatin gene transfer on subcutaneous and intraperitoneal growth of murine tumors. Kalluri R: Basement membranes: structure, assembly and role in tumour angiogenesis. Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R: Distinct antitumor properties of a type IV collagen domain derived from basement membrane. Maeshima Y, Colorado PC, Kalluri R: Two RGD-independent alpha vbeta 3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, Kharbanda S, Kalluri R: Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R: Anti-angiogenic cues from vascular basement membrane collagen. Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R: Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. Xu R, Yao ZY, Xin L, Zhang Q, Li TP, Gan RB: NC1 domain of human type VIII collagen alpha 1 inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Biochem Biophys Res Commun. Ramchandran R, Dhanabal M, Volk R, Waterman MJ, Segal M, Lu H, Knebelmann B, Sukhatme VP: Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Cao Y, Moses M, Lane WS, Sage EH, Folkman J: Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb Symp Quant Biol. O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J: Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. O'Reilly MS, Holmgren L, Chen C, Folkman J: Angiostatin induces and sustains dormancy of human primary tumors in mice. Wahl ML, Moser TL, Pizzo SV: Angiostatin and Anti-angiogenic Therapy in Human Disease. Recent Prog Horm Res. Beerepoot LV, Witteveen EO, Groenewegen G, Fogler WE, Sim BK, Sidor C, Zonnenberg BA, Schramel F, Gebbink MF, Voest EE: Recombinant human angiostatin by twice-daily subcutaneous injection in advanced cancer: a pharmacokinetic and long-term safety study. Clin Cancer Res. Lalani AS, Chang B, Lin J, Case SS, Luan B, Wu-Prior WW, VanRoey M, Jooss K: Anti-tumor efficacy of human angiostatin using liver-mediated adeno-associated virus gene therapy. Mol Ther. Galaup A, Opolon P, Bouquet C, Li H, Opolon D, Bissery MC, Tursz T, Perricaudet M, Griscelli F: Combined effects of docetaxel and angiostatin gene therapy in prostate tumor model. Clapp C, Martial JA, Guzman RC, Rentier-Delure F, Weiner RI: The kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Tabruyn SP, Sorlet CM, Rentier-Delrue F, Bours V, Weiner RI, Martial JA, Struman I: The antiangiogenic factor 16K human prolactin induces caspase-dependent apoptosis by a mechanism that requires activation of nuclear factor-kappaB. Mol Endocrinol. Kim J, Luo W, Chen DT, Earley K, Tunstead J, Yu-Lee LY, Lin SH: Antitumor activity of the kDa prolactin fragment in prostate cancer. Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ: Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Sharpe RJ, Byers HR, Scott CF, Bauer SI, Maione TE: Growth inhibition of murine melanoma and human colon carcinoma by recombinant human platelet factor 4. Kolber DL, Knisely TL, Maione TE: Inhibition of development of murine melanoma lung metastases by systemic administration of recombinant platelet factor 4. Belman N, Bonnem EM, Harvey HA, Lipton A: Phase I trial of recombinant platelet factor 4 rPF4 in patients with advanced colorectal carcinoma. Invest New Drugs. Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA: Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Li Y, Jin Y, Chen H, Jie G, Tobelem G, Caen JP, Han ZC: Suppression of tumor growth by viral vector-mediated gene transfer of N-terminal truncated platelet factor 4. Cancer Biother Radiopharm. Neville LF, Mathiak G, Bagasra O: The immunobiology of interferon-gamma inducible protein 10 kD IP : a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. Feldman AL, Friedl J, Lans TE, Libutti SK, Lorang D, Miller MS, Turner EM, Hewitt SM, Alexander HR: Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer. Jones PF: Not just angiogenesis--wider roles for the angiopoietins. Lin P, Buxton JA, Acheson A, Radziejewski C, Maisonpierre PC, Yancopoulos GD, Channon KM, Hale LP, Dewhirst MW, George SE, Peters KG: Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Trinchieri G: Interleukin and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J: Inhibition of angiogenesis in vivo by interleukin Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK: Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. Portielje JE, Kruit WH, Schuler M, Beck J, Lamers CH, Stoter G, Huber C, de Boer-Dennert M, Rakhit A, Bolhuis RL, Aulitzky WE: Phase I study of subcutaneously administered recombinant human interleukin 12 in patients with advanced renal cell cancer. Gollob JA, Mier JW, Veenstra K, McDermott DF, Clancy D, Clancy M, Atkins MB: Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-gamma induction is associated with clinical response. Hurteau JA, Blessing JA, DeCesare SL, Creasman WT: Evaluation of recombinant human interleukin in patients with recurrent or refractory ovarian cancer: a gynecologic oncology group study. Gynecol Oncol. Mazzolini G, Prieto J, Melero I: Gene therapy of cancer with interleukin Caruso M, Pham-Nguyen K, Kwong YL, Xu B, Kosai KI, Finegold M, Woo SL, Chen SH: Adenovirus-mediated interleukin gene therapy for metastatic colon carcinoma. Cao R, Farnebo J, Kurimoto M, Cao Y: Interleukin acts as an angiogenesis and tumor suppressor. Faseb J. Nagai H, Hara I, Horikawa T, Oka M, Kamidono S, Ichihashi M: Gene transfer of secreted-type modified interleukin gene to B16F10 melanoma cells suppresses in vivo tumor growth through inhibition of tumor vessel formation. J Invest Dermatol. Liu Y, Huang H, Saxena A, Xiang J: Intratumoral coinjection of two adenoviral vectors expressing functional interleukin and inducible protein, respectively, synergizes to facilitate regression of established tumors. Sidky YA, Borden EC: Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Dvorak HF, Gresser I: Microvascular injury in pathogenesis of interferon-induced necrosis of subcutaneous tumors in mice. Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ: Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Lindner DJ: Interferons as antiangiogenic agents. Curr Oncol Rep. Albini A, Marchisone C, Del Grosso F, Benelli R, Masiello L, Tacchetti C, Bono M, Ferrantini M, Rozera C, Truini M, Belardelli F, Santi L, Noonan DM: Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: A gene therapy approach. Berger AC, Alexander HR, Tang G, Wu PS, Hewitt SM, Turner E, Kruger E, Figg WD, Grove A, Kohn E, Stern D, Libutti SK: Endothelial monocyte activating polypeptide II induces endothelial cell apoptosis and may inhibit tumor angiogenesis. Microvasc Res. Wu PC, Alexander HR, Huang J, Hwu P, Gnant M, Berger AC, Turner E, Wilson O, Libutti SK: In vivo sensitivity of human melanoma to tumor necrosis factor TNF -alpha is determined by tumor production of the novel cytokine endothelial-monocyte activating polypeptide II EMAPII. Gnant MF, Berger AC, Huang J, Puhlmann M, Wu PC, Merino MJ, Bartlett DL, Alexander H. Jiang Y, Goldberg ID, Shi YE: Complex roles of tissue inhibitors of metalloproteinases in cancer. Wang M, Liu YE, Greene J, Sheng S, Fuchs A, Rosen EM, Shi YE: Inhibition of tumor growth and metastasis of human breast cancer cells transfected with tissue inhibitor of metalloproteinase 4. ten Hagen TL, Eggermont AM: Solid tumor therapy: manipulation of the vasculature with TNF. Technol Cancer Res Treat. Senzer N, Mani S, Rosemurgy A, Nemunaitis J, Cunningham C, Guha C, Bayol N, Gillen M, Chu K, Rasmussen C, Rasmussen H, Kufe D, Weichselbaum R, Hanna N: TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. Grant SW, Kyshtoobayeva AS, Kurosaki T, Jakowatz J, Fruehauf JP: Mutant p53 correlates with reduced expression of thrombospondin-1, increased angiogenesis, and metastatic progression in melanoma. Cancer Detect Prev. Van Meir EG, Polverini PJ, Chazin VR, Su Huang HJ, de Tribolet N, Cavenee WK: Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nat Genet. Holmgren L, Jackson G, Arbiser J: p53 induces angiogenesis-restricted dormancy in a mouse fibrosarcoma. Su JD, Mayo LD, Donner DB, Durden DL: PTEN and phosphatidylinositol 3'-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Szary J, Szala S: Intra-tumoral administration of naked plasmid DNA encoding mouse endostatin inhibits renal carcinoma growth. Oga M, Takenaga K, Sato Y, Nakajima H, Koshikawa N, Osato K, Sakiyama S: Inhibition of metastatic brain tumor growth by intramuscular administration of the endostatin gene. Int J Oncol. Blezinger P, Wang J, Gondo M, Quezada A, Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R, Min W: Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene. Nat Biotechnol. Barron LG, Uyechi LS, Szoka F. Gene Ther. Marty C, Odermatt B, Schott H, Neri D, Ballmer-Hofer K, Klemenz R, Schwendener RA: Cytotoxic targeting of F9 teratocarcinoma tumours with anti-ED-B fibronectin scFv antibody modified liposomes. Br J Cancer. Templeton NS, Lasic DD, Frederik PM, Strey HH, Roberts DD, Pavlakis GN: Improved DNA: liposome complexes for increased systemic delivery and gene expression. Rodolfo M, Cato EM, Soldati S, Ceruti R, Asioli M, Scanziani E, Vezzoni P, Parmiani G, Sacco MG: Growth of human melanoma xenografts is suppressed by systemic angiostatin gene therapy. Chen QR, Kumar D, Stass SA, Mixson AJ: Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, Hanahan D, McDonald DM: Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J Clin Invest. Kondo M, Asai T, Katanasaka Y, Sadzuka Y, Tsukada H, Ogino K, Taki T, Baba K, Oku N: Anti-neovascular therapy by liposomal drug targeted to membrane type-1 matrix metalloproteinase. Janssen AP, Schiffelers RM, ten Hagen TL, Koning GA, Schraa AJ, Kok RJ, Storm G, Molema G: Peptide-targeted PEG-liposomes in anti-angiogenic therapy. Int J Pharm. Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M: Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Gu ZP, Wang YJ, Li JG, Zhou YA: VEGF antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol. Im SA, Gomez-Manzano C, Fueyo J, Liu TJ, Ke LD, Kim JS, Lee HY, Steck PA, Kyritsis AP, Yung WK: Antiangiogenesis treatment for gliomas: transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Marchand GS, Noiseux N, Tanguay JF, Sirois MG: Blockade of in vivo VEGF-mediated angiogenesis by antisense gene therapy: role of Flk-1 and Flt-1 receptors. Am J Physiol Heart Circ Physiol. Shim WS, Teh M, Mack PO, Ge R: Inhibition of angiopoietin-1 expression in tumor cells by an antisense RNA approach inhibited xenograft tumor growth in immunodeficient mice. Dallabrida SM, De Sousa MA, Farrell DH: Expression of antisense to integrin subunit beta 3 inhibits microvascular endothelial cell capillary tube formation in fibrin. Lipscomb EA, Dugan AS, Rabinovitz I, Mercurio AM: Use of RNA interference to inhibit integrin alpha6beta4 -mediated invasion and migration of breast carcinoma cells. Clin Exp Metastasis. Yang G, Cai KQ, Thompson-Lanza JA, Bast R. Brummelkamp TR, Bernards R, Agami R: A system for stable expression of short interfering RNAs in mammalian cells. Zhang L, Yang N, Mohamed-Hadley A, Rubin SC, Coukos G: Vector-based RNAi, a novel tool for isoform-specific knock-down of VEGF and anti-angiogenesis gene therapy of cancer. Thomas CE, Ehrhardt A, Kay MA: Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. Reynolds PN, Zinn KR, Gavrilyuk VD, Balyasnikova IV, Rogers BE, Buchsbaum DJ, Wang MH, Miletich DJ, Grizzle WE, Douglas JT, Danilov SM, Curiel DT: A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Yotnda P, Chen DH, Chiu W, Piedra PA, Davis A, Templeton NS, Brenner MK: Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Logg CR, Kasahara N: Retrovirus-mediated gene transfer to tumors: utilizing the replicative power of viruses to achieve highly efficient tumor transduction in vivo. Methods Mol Biol. Rollins SA, Birks CW, Setter E, Squinto SP, Rother RP: Retroviral vector producer cell killing in human serum is mediated by natural antibody and complement: strategies for evading the humoral immune response. Hum Gene Ther. Ghazizadeh S, Carroll JM, Taichman LB: Repression of retrovirus-mediated transgene expression by interferons: implications for gene therapy. J Virol. Smith-Arica JR, Bartlett JS: Gene therapy: recombinant adeno-associated virus vectors. Curr Cardiol Rep. White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED, Hallek M, Baker AH: Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Giles J: Protests win reprieve for renowned medical lab. Kay MA, Nakai H: Looking into the safety of AAV vectors. Shichinohe T, Bochner BH, Mizutani K, Nishida M, Hegerich-Gilliam S, Naldini L, Kasahara N: Development of lentiviral vectors for antiangiogenic gene delivery. Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM: Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. Latchman DS: Herpes simplex virus vectors for gene therapy. Mol Biotechnol. Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ: Cross-packaging of a single adeno-associated virus AAV type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. Khare PD, Liao S, Hirose Y, Kuroki M, Fujimura S, Yamauchi Y, Miyajima-Uchida H: Tumor growth suppression by a retroviral vector displaying scFv antibody to CEA and carrying the iNOS gene. Anticancer Res. Reynolds PN, Nicklin SA, Kaliberova L, Boatman BG, Grizzle WE, Balyasnikova IV, Baker AH, Danilov SM, Curiel DT: Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Varda-Bloom N, Shaish A, Gonen A, Levanon K, Greenbereger S, Ferber S, Levkovitz H, Castel D, Goldberg I, Afek A, Kopolovitc Y, Harats D: Tissue-specific gene therapy directed to tumor angiogenesis. Thorpe PE: Vascular targeting agents as cancer therapeutics. Tozer GM, Kanthou C, Parkins CS, Hill SA: The biology of the combretastatins as tumour vascular targeting agents. Int J Exp Pathol. Pasqualini R, Koivunen E, Ruoslahti E: Alpha v integrins as receptors for tumor targeting by circulating ligands. Arap W, Pasqualini R, Ruoslahti E: Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E: A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R: Tumor targeting with a selective gelatinase inhibitor. Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. A recent study 7 has identified that the expression of a number of genes is increased in the tumor endothelium when compared with the nontumor endothelial cells. Thus, because the tumor endothelium appears to have a number of altered features, the best candidate antiangiogenic molecules for cancer therapy should be directed at differences between the tumor vasculature and normal vasculature to increase specificity and efficacy. However, many of the current antiangiogenic candidates also regulate similar activities, not only in tumor endothelium but also in normal endothelium and even in nontransformed cell types. The effectiveness of angiogenesis inhibitors in the treatment of various cancers remains to be determined. Despite the possibility of tumor endothelium being heterogeneous and tumor type specific, antiangiogenesis inhibitors have been used in some 22 clinical trials to date. However, a major problem has been the availability amount and purity and the stability of some of these molecules. Although there have been promising results in animal studies, the human trials for these protein-based inhibitors have been less successful 8. The questions of how best to deliver the inhibitor, maintain its stability and activity, and target it to the tumor vasculature have not been answered. Gene therapy represents an interesting alternative for the effective delivery of antiangiogenic therapy, and a number of studies 9 — 16 have demonstrated that a gene therapy-based antiangiogenesis approach is an effective means of reducing tumor growth in animal models. Advantages of gene therapy over the direct administration of the inhibitors include the localized delivery and sustained expression of the antiangiogenic molecules, the ability to inhibit multiple angiogenic pathways with the delivery of more than one transgene, the generation of properly folded inhibitor molecules, and the potential for decreased cost 8 , Endostatin, a kd fragment of collagen XVIII with demonstrated antiangiogenic activity, is being tested in phase I clinical trials as a protein infusion for various cancers. The gene for this protein is an ideal gene therapy candidate because the recombinant protein is difficult to produce and appears to be safe when delivered to the patient for an extended time. Endostatin has been tested in murine tumor models, with varying success, by gene therapy delivery vectors, including adenoviral vectors, adeno-associated viral vectors, in vitro transfections, polymerized plasmids, and DNA cationic liposomes 9 — 13 , In this issue of the Journal, Feldman et al. They found that endostatin gene transfer led to the expression of functional endostatin and inhibited tumor growth. This study highlights a number of important points to consider in antiangiogenesis gene therapy. First, there is no correlation between efficacy and the level of endostatin in the serum. For example, in the study by Feldman et al. One possibility for this latter observation is that effective inhibition of endothelial activity is dependent on both the ability to deliver endostatin to the right location and the response of the endothelial cells within the local tumor microenvironment. Second, a lack of understanding of the mechanism s of action of endostatin has greatly hindered current efforts to improve its efficacy in vivo. In this respect, two recent studies 17 , 18 provide compelling data that a primary action of endostatin is the disruption of appropriate interactions between cell surface receptors and extracellular matrix proteins. Currently, with the mechanism of action of a large number of putative antiangiogenic molecules unclear, any efforts to translate these research findings to clinical therapies may be premature. Finally, the study by Feldman et al. However, retroviral vectors only transduce dividing cells, which may limit their in vivo use. Such limitations highlight the continued need for improved gene therapy vectors to overcome the current problems associated with various gene therapy vectors, including immune response to the vector, transduction efficiency, delivery of the gene to the target tissue, and controlled gene expression by the vector. The general area of angiogenesis modulation is an exciting one, and gene therapy directed to inhibit angiogenesis can benefit from the more advanced gene therapy efforts to promote neovascularization 8. The early indications of success suggest that modulation of angiogenesis via gene therapy is reasonably safe and effective and offers considerable insight into the criteria for successful antiangiogenesis therapy. First, effective therapeutic angiogenesis is based on the considerable knowledge of how VEGF and FGF work. Second, it is likely that multiple factors will be necessary to generate vessels that are stable over time. Third, it remains unclear what is the best vector system to deliver optimal therapy. For researchers to realize the considerable advantages of antiangiogenesis gene therapy, it will be necessary to develop better vector platforms. Additional challenges for antiangiogenesis therapy are to understand how angiogenesis inhibitors function, how tumor vessels differ from normal blood vessels, and how to target tumor vessels with appropriate combination therapies. Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol ; 33 : — Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature ; : — Jung YD, Ahmad SA, Akagi Y, Takahashi Y, Liu W, Reinmuth N, et al. Role of the tumor microenvironment in mediating response to anti-angiogenic therapy. Cancer Metastasis Rev ; 19 : — Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. Clin Invest ; : — Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol ; : — Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A ; 97 : — St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science ; : — Liau G, Su EJ, Dixon KD. Clinical efforts to modulate new blood vessel formation in the adult; a comparison of gene therapy versus conventional approaches. Drug Discovery Today ; 6 : 19 — Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A ; 98 : — Jia S, Shu F, Li H, Fushu H, Ziu R. Clin Hemorheology Microcircul ; 23 : —7. Chen CT, Lin J, Li Q, Phipps SS, Jakubczak JL, Stewart DA, et al. Antiangiogenic gene therapy for cancer via systemic administration of adenoviral vectors expressing secretable endostatin. Hum Gene Ther ; 11 : — Ding I, Sun JZ, Fenton B, Liu WM, Kimsely P, Okunieff P, et al. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res ; 61 : — Szary J, Szala S. Intra-tumoral administration of naked plasmid DNA encoding mouse endostatin inhibits renal carcinoma growth. Int J Cancer ; 91 : —9. Regulier E, Pau S, Marigliano M, Kintz J, Poitevin Y, Ledoux C, et al. Adenovirus-mediated delivery of antiangiogenic genes as an antitumor approach. Cancer Gene Ther ; 8 : 45 — Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci U S A ; 97 : —7. Feldman AL, Alexander HR, Hewitt SM, Lorang D, Thiruvathukal CE, Turner EM, et al. Effect of retroviral endostatin gene transfer on subcutaneous and intraperitoneal growth of murine tumors. J Natl Cancer Inst ; 93 : — Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo CR, et al. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci U S A ; 98 : —9. Kuo CJ, LaMontagne KR Jr, Garcia-Cardena G, Ackley BD, Kalman D, et al. J Cell Biol ; : — Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross R, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency SCID -X1 disease. Science ; : —9. Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis. Issues, problems, consensus: An expert panel summary. Circulation ; : E73 — Webster KA. Therapeutic angiogenesis: a case for targeted, regulated gene delivery. |
| Antiangiogenic gene therapy of cancer: recent developments | Chitosan is another carrier derived from chitin. It is less cytotoxic and is biodegradable and metabolized easily by the kidneys. In mice models of breast cancer, chitosan nanoparticles containing anti-Rho small interfering RNA siRNA showed tumour anti-angiogenesis [ 56 ]. The binding of αvβ3 integrin to chitosan nanoparticles is an important development. The receptor for αvβ3 integrin is widely expressed in tumours and has shown potentials in ovarian cancer models. Encapsulation of paclitaxel with chitosan nanoparticles has shown efficacy in breast cancer [ 57 ]. There is now interest in the use of mesenchymal stem cells MSCs to deliver nanoparticles. Hypoxic conditioning of such MSCs used as cell-based therapy can be used for aggressive tumours like glioblastoma multiforme since MSCs can traffic across the blood-brain barrier [ 53 ]. Blocking tumour stem cells via anti-angiogenic therapies is another theoretical approach since the tumour stem cell sub-population in some tumours like breast cancers may be more adept at promoting angiogenesis than their non-stem cell counterparts. The different delivery methods for nanoparticles are compared in Table 2. Anti-angiogenic therapy in cancers has enormous potentials using VEGF signaling pathways. Cardiovascular toxicity and off-target effects of anti-angiogenic drugs are impediments to their long-term use in those at high cardiovascular risk. Continued research into effective nanoparticle-based delivery methods is an exciting and developing field in cancer therapeutics. Understanding of the molecular and cellular mechanisms of tumour angiogenesis will facilitate the development of newer effective anti-angiogenic molecules. GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for diseases and injuries for countries, — a systematic analysis for the global burden of disease study Google Scholar. Gupta K, Zhang J. Angiogenesis: a curse or cure? Postgrad Med J. Article CAS PubMed PubMed Central Google Scholar. Kim KJ, Li B, Winer B, Armanini M, Gillett N, Philips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nat Publ Gr. CAS Google Scholar. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. Article CAS PubMed Google Scholar. Planchard D, Planchard D. Bevacizumab in non-small-cell lung cancer: a review. Expert Rev Anticancer Ther. Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, et al. Cancer Res. Liu L, Cao Y, Chen C, Zhang X, Mcnabola A, Wilkie D, et al. Chase DM, Chaplin DJ, Monk BJ. The development and use of vascular targeted therapy in ovarian cancer. Gynecol Oncol. Kazazi-Hyseni F, Beijnen JH, Schellens JH. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Miles DW, Chan A, Dirix LY, Corte J. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2 — negative metastatic breast cancer. J Clin Oncol. Robert NJ, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, et al. RIBBON randomized, double-blind, placebo-controlled, phase iii trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Prausova J, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non—small-cell lung cancer: a randomized, controlled phase III trial. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine RAISE : a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: the great discovery and greater complexity review. Int J Oncol. Li J-L, Sainson RCA, Oon CE, Turley H, Leek R, Sheldon H, et al. DLL4-notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. Jeong W, Rapisarda A, Ryun S, Robert P, Chen A, Melillo G, et al. Pilot trial of EZN, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha HIF-1α , in patients with refractory solid tumors. Cancer Chemother Pharmacol. Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Van Cutsem E, Nanayakkara N, et al. Phase II randomized, double-blind, placebo-controlled study of AMG trebananib in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann Oncol. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-Angiogenic therapy and development of third-generation anti-Angiogenic drug candidates. Genes Cancer. Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Shojaei F, Ferrara N. Drug Resist Updat. Orimo A, Gupta PB, Sgroi DC, Arenzana-seisdedos F, Delaunay T, Naeem R, et al. Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science Article CAS Google Scholar. Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Acquired tumor resistance to antiangiogenic therapy: mechanisms at a glance. J Res Med Sci. Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy. Cold Spring Harb Perspect Med. Article Google Scholar. Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Article PubMed PubMed Central Google Scholar. Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 VEGFR2 induces synergistic anti-tumour effectin vivo. Clin Exp Immunol. Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Hillan K, Koeppen K, Tobin P, Pham T. The role of VEGF expression in response to bevacizumab plus capecitabine in metastatic breast cancer MBC. Proc Am Soc Clin Oncol. Escudier B, Eisen T, Stadler WM, Szczylik C, Demkow T, Hutson TE, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase iii treatment approaches in renal cancer global evaluation trial. Reinmuth N, Thomas M, Meister M, Schnabel PA, Kreuter M. Current data on predictive markers for anti-angiogenic therapy in thoracic tumours. Eur Respir J. Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, et al. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Martinetti A, Miceli R, Sottotetti E, Di Bartolomeo M, De Braud F, Gevorgyan A, et al. Circulating biomarkers in advanced colorectal cancer patients randomly assigned to three bevacizumab-based regimens. Cancers Basel. Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-neblett KL, Martin A, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Sammarco G, Gallo G, Vescio G, Picciariello A, Paola DG, Trompetto M, et al. Mast cells, micrornas and others: the role of translational research on colorectal cancer in the forthcoming era of precision medicine. J Clin Med. Ammendola M, Sacco R, Sammarco G, Luposella M, Patruno R, Gadaleta COD, et al. Mast cell-targeted strategies in cancer therapy. Transfus Med Hemother. Angelucci A, Di Padova M. Int J Mol Sci. Meert A-P, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout J-M, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with. Br J Cancer. Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al. Impact of vascular endothelial growth factor-a expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. Shiroishi MS, Boxerman JL, Pope WB. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Rojas JD, Lin F, Chiang Y, Chytil A, Chong DC, Bautch VL, et al. Ultrasound molecular imaging of VEGFR-2 in clear-cell renal cell carcinoma tracks disease response to antiangiogenic and notch-inhibition therapy. Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. Touyz RM, Lang NN. Hypertension and antiangiogenesis the Janus face of VEGF inhibitors. JACC Cardio Oncol. Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. de la Torre P, Pérez-Lorenzo MJ, Alcázar-garrido Á, Flores AI. Cell-based nanoparticles delivery systems for targeted cancer therapy: lessons from anti-angiogenesis treatments. Mukherjee S, Patra CR. Therapeutic application of anti-angiogenic nanomaterials in cancers. Liu H, Zhang Y, Zheng S, Weng Z, Ma J, Li Y, et al. Biochemical and biophysical research communications detention of copper by sulfur nanoparticles inhibits the proliferation of A malignant melanoma and MCF-7 breast cancer cells. Biochem Biophys Res Commun. Potdar PD, Shetti AU. Chitosan nanoparticles: an emerging weapon against the cancer. MOJ Cell Sci Rep. Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS PharmSciTech. Download references. Nuffield Department of Population Health, University of Oxford, Oxford, UK. Institute of Cardiovascular Science, University College London, London, UK. Ayodipupo S. Department of Basic Science, Prince Sultan Bin Abdulaziz College for Emergency Medical Services, King Saud University, Riyadh, Saudi Arabia. You can also search for this author in PubMed Google Scholar. ASO conceptualized the topic, designed the study methodology, conducted the literature search, and wrote the initial draft. FA, MA, AA and MB conceptualized the topic, conducted the literature search and contributed to the initial draft. The authors read and approved the final draft of the manuscript and take responsibility for this paper. Correspondence to Ayodipupo S. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Oguntade, A. et al. Anti-angiogenesis in cancer therapeutics: the magic bullet. J Egypt Natl Canc Inst 33 , 15 Download citation. Received : 18 November Accepted : 08 June Published : 02 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all SpringerOpen articles Search. Download PDF. Narrative Review Open access Published: 02 July Anti-angiogenesis in cancer therapeutics: the magic bullet Ayodipupo S. Oguntade ORCID: orcid. Abstract Background Angiogenesis is the formation of new vascular networks from preexisting ones through the migration and proliferation of differentiated endothelial cells. Main body of the abstract MEDLINE and EMBASE databases were searched for publications on antiangiogenic therapy in cancer therapeutics from to Short conclusion Clinical surveillance is important for the early detection of tumour resistance and treatment failure using reliable biomarkers. Background Cancers still account for significant morbidity and mortality globally despite remarkable advances in the management of cancers [ 1 ]. Main text We searched MEDLINE and EMBASE for publications on anti-angiogenesis in cancer from to as part of a larger project on anti-angiogenesis and cancer therapeutics. Anti-angiogenics in cancers Several preclinical and clinical studies in cancer research have targeted different steps of the angiogenic pathway. Table 1 Selected VEGF-targeted anti-angiogenics and their therapeutic indications Full size table. Clinical approach to cardiovascular toxicity of antiangiogenic therapy. Full size image. Table 2 Different delivery methods for nanoparticles Full size table. Conclusion Anti-angiogenic therapy in cancers has enormous potentials using VEGF signaling pathways. Availability of data and materials Not applicable. References GBD Disease and Injury Incidence and Prevalence Collaborators. Google Scholar Gupta K, Zhang J. Article CAS PubMed PubMed Central Google Scholar Kim KJ, Li B, Winer B, Armanini M, Gillett N, Philips HS, et al. CAS Google Scholar Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Article CAS PubMed Google Scholar Planchard D, Planchard D. Article CAS PubMed Google Scholar Shih T, Lindley C. Article CAS PubMed Google Scholar Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, et al. Article CAS PubMed Google Scholar Chase DM, Chaplin DJ, Monk BJ. Article CAS PubMed Google Scholar Kazazi-Hyseni F, Beijnen JH, Schellens JH. Article CAS PubMed PubMed Central Google Scholar Ferrara N, Kerbel RS. Article CAS PubMed Google Scholar Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Article CAS PubMed Google Scholar Miles DW, Chan A, Dirix LY, Corte J. Article CAS PubMed Google Scholar Robert NJ, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, et al. Article CAS PubMed Google Scholar Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Prausova J, et al. Article CAS PubMed Google Scholar Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Article CAS PubMed Google Scholar Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-carbonero R, et al. Article CAS PubMed Google Scholar Maj E, Papiernik D, Wietrzyk J. Article CAS PubMed PubMed Central Google Scholar Li J-L, Sainson RCA, Oon CE, Turley H, Leek R, Sheldon H, et al. Article CAS PubMed Google Scholar Clarke JM, Hurwitz HI. Article CAS PubMed PubMed Central Google Scholar Balamurugan K. Article CAS PubMed Google Scholar Jeong W, Rapisarda A, Ryun S, Robert P, Chen A, Melillo G, et al. Article CAS PubMed Google Scholar Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Van Cutsem E, Nanayakkara N, et al. Article CAS PubMed Google Scholar Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Article CAS PubMed PubMed Central Google Scholar Loges S, Schmidt T, Carmeliet P. Article CAS PubMed PubMed Central Google Scholar Viallard C, Larrivee B. Article CAS PubMed Google Scholar Shojaei F, Ferrara N. Article CAS PubMed Google Scholar Orimo A, Gupta PB, Sgroi DC, Arenzana-seisdedos F, Delaunay T, Naeem R, et al. Article CAS PubMed Google Scholar Guo W, Giancotti FG. Article CAS PubMed Google Scholar Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Article CAS Google Scholar Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Article CAS Google Scholar Goel S, Wong AH, Jain RK. Article Google Scholar Ramjiawan RR, Griffioen AW, Duda DG. Article PubMed PubMed Central Google Scholar Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Article CAS PubMed PubMed Central Google Scholar Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Article PubMed PubMed Central Google Scholar Hillan K, Koeppen K, Tobin P, Pham T. Google Scholar Escudier B, Eisen T, Stadler WM, Szczylik C, Demkow T, Hutson TE, et al. Article CAS PubMed Google Scholar Reinmuth N, Thomas M, Meister M, Schnabel PA, Kreuter M. Article CAS PubMed Google Scholar Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, et al. Article CAS PubMed Google Scholar Martinetti A, Miceli R, Sottotetti E, Di Bartolomeo M, De Braud F, Gevorgyan A, et al. Article Google Scholar Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-neblett KL, Martin A, et al. Article CAS PubMed Google Scholar Sammarco G, Gallo G, Vescio G, Picciariello A, Paola DG, Trompetto M, et al. Article Google Scholar Ammendola M, Sacco R, Sammarco G, Luposella M, Patruno R, Gadaleta COD, et al. Article PubMed PubMed Central Google Scholar Angelucci A, Di Padova M. Google Scholar Meert A-P, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout J-M, et al. Article PubMed PubMed Central Google Scholar Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al. Article CAS PubMed Google Scholar Shiroishi MS, Boxerman JL, Pope WB. Article CAS PubMed Google Scholar Vasudev NS, Reynolds AR. Article CAS PubMed PubMed Central Google Scholar Rojas JD, Lin F, Chiang Y, Chytil A, Chong DC, Bautch VL, et al. Article CAS PubMed PubMed Central Google Scholar Touyz RM, Herrmann J. Article Google Scholar Touyz RM, Lang NN. Article Google Scholar Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Electrically mediated delivery of vector plasmid DNA elicits an antitumor effect. Liu F, Huang L. Electric gene transfer to the liver following systemic administration of plasmid DNA. Sanz G, Satkauskas S, Mir LM. In vivo DNA electrotransfer in skeletal muscle. Methods Mol Biol ; : — Hagstrom JE. Plasmid-based gene delivery to target tissues in vivo : the intravascular approach. Curr Opin Mol Ther ; 5 : — Herweijer H, Wolff JA. Progress and prospects: naked DANN gene transfer and therapy. Gene Therapy ; 10 : — Gollins H, McMahon J, Wells KE, Wells DJ. High-efficiency plasmid gene transfer into dystrophic muscle. Smith AE. Viral vectors in gene therapy. Annu Rev Microbiol ; 49 : — Buttrick PM. Gene therapy in the cardiovascular system: current strategies and practical limitations. J Am Coll Cardiol ; 6 : 17— Templeton NS. Liposomal delivery of nucleic acids in vivo. DNA and Cell Biol ; 21 : — Ferrara N, Alitalo K. Clinical application of angiogenic growth factors and their inhibitors. Nat Med ; 5 : — Hamaway AH, Lee LY, Crystal RG, Rosengart TK. Cardiac angiogenesis and gene therapy: a strategy for myocardial revascularization. Curr Opin Cardiol ; 14 : — Rosengart TK, Patel SR, Crystal RG. Therapeutic angiogenesis: proteine and gene therapy delivery strategies. J Cardiovasc Risk ; 6 : 29— Abo-Auda W, Benza RL. Therapeutic angiogenesis: review of current concepts and future directions. J Heart Lung Transplantation ; 22 : — Unger EF, Banai S, Shou M. Basic fibroblast growth factor enhances myocardial collateral flow in canine model. Am J Physiol ; : H—H Baffour R et al. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in rabbit model of acute lower limb ischemia: dose—response effect of basic fibroblast growth factor. J Vasc Surg ; 16 : — Banai S, Jaklitsch MT, Shou M. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Ciculation ; 89 : — Hariawala MD, Horowitz JR, Esakof D. VEGF improves myocardial blood flow but produces EDRF-mediated hypotension in porcine hearts. J Surg Res ; 63 : 77— Lopez JJ, Laham RJ, Stamler A. VEGF administration in chronic myocardial ischemia in pigs. Cardiovasc Res ; 40 : — Kornowski R, Fuchs S, Leon M, Epstein SE. Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation ; : — Laham RJ, Leimbach M, Chronos NA. Intracoronary administration of recombinant fibroblast growth factor-2 in patients with severe CAD: results of phase I. J Am Coll Cardiol ; 33 Suppl A : A. Laham RJ, Sellke FW, Edelman ER. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary by pass surgery: results of phase I randomized double blind, placebo-controlled trial. Scumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation ; 97 : — Laham RJ, Selke FW, Edelman ER. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary by pass surgery: results of phase I randomized double-blind, placebo controlled trial. Hendel RC, Henry TD, Roha-Singh K. Effect of intracoronary recombinant human vascular endothelial growth factor rhVEGF on myocardial perfusion: evidence for a dose dependent effect. Henry TD, Annes BH, Azrin MA. Double-blind placebo controlled trial of recombinant human vascular endothelial growth factor: the VIVA trial. Henry TD, Mckendall GR, Azrin MA. VIVA trial: one year follow up. Circulation ; Suppl II : Nicklin SA, Baker AH. Development of targeted viral vectors for cardiovascular gene therapy. Genet Eng NY ; 25 : 15— Tsurumi Y, Takeshita S, Chen D. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation ; 94 : — Wolff JA, Malone RW, Williams P. Direct gene transfer into mouse muscle in vivo. Thompson RB, Rungwerth K, Koch WJ. Gene therapy for heart failure. Ann Med ; 36 Suppl 1 : — Mack ChA, Patel SR, Schwarz EA, Zanzonico P. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor improves myocardial perfusion in the ischemic porcine heart. J Thorac Cardiovasc Surg ; : — Asahara T, Bauters Ch, Zeng LuP, Takeshita S. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation ; 92 : — Kondoh K, Koyama H, Miyata T, Takato T. Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. Cardiovasc Res ; 61 : — Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci USA ; 97 : — Isner JM, Pieczek A, Schainfeld R. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF in patients with ischemic limb. Baugmgartner I, Pieczek A, Manor O. Constitutive expression of phVEGF after intramuscular gene transfer promotes collateral vessel developementin patients with critical ischemia. Losordo DW, Vale PR, Symes JF. Gene therapy for myocardial angiogenesis; initial clinical results with direct myocardial injection of phVEGF as a sole therapy for myocardial ischemia. Circulation ; 98 : — Rosengart TK, Lee LY, Patel SR, Sanborn TA. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VAGF cDNA to individuals with clinically significant severe coronary artery disease. Kolsut P et al. Gene therapy of coronary artery disease with phvegf — early outcome. Polish Heart J ; 59 : — Vale PR et al. Randomised, single-blind, placebo controlled pilot study catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Grines C, Rubanyi GM, Kleiman NS, Marrot P. Angiogenic gene therapy with adenovirus 5 fibroblast growth factor-4 Ad5FGF-4 : a new option for the treatment of coronary artery disease. Am J Cardiol ; 92 Suppl : 24N—31N. Malecki M, Przybyszewska M, Janik P. Construction of a bicistronic proangiogenic expression vector and its application in the experimental angiogenesis in vivo. Acta Biochim Pol ; 50 : — Ibukiyama C. Angiogenic therapy using fibroblast growth factor and vascular endothelial growth factor for ischemic vascular lesions. Jpn Heart J ; 37 : — Whitlock PR et al. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol Ther ; 9 : 67— Cines DB, Pollak ES, Buck CA. Endothelial cell in physiology and in the pathophysiology of vascular disorders. Blood ; 91 : — Kessler P. Biobypass, ready for the NOVA Trial. NOGA Lett ; 5 : 2—4. Yamauchi A et al. Pre-administration of angiopoietin-1 followed by VEGF induces functional and mature vascular formation in a rabbit ischemic model. J Gene Med ; 5 : — Isner JM et al. Arterial gene transfer for therapeutic angiogenesis in patients with peripheral artery disease. Hum Gene Ther ; 7 : — Treatment of tromboangiitis obliterans Buerger's disease by intramuscular gene transfer of VEGF: preliminary clinical results. J Vasc Surg ; 28 : — Baumgartner I et al. Lower-extremity edema associated with gene transfer of naked DNA encoding vascular endothelial growth factor. Ann Intern Med ; : — Ozaki H et al. Blockade of vascular endothelial growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol ; : — Celletti FL et al. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med ; 7 : — van Weel V et al. Vascular endothelial growth factor overexpression in ischemic skeletal muscle enhances myoglobin expression in vivo. Circ Res ; 95 : 58— Mohler ER et al. Adenoviral-mediated gene transfer of vascular endothelial growth factor in critical limb ischemia: safety results from a phase I trial. Vasc Med ; 8 : 9— Download references. Department of Cell Biology, Centre of Oncology, Maria Sklodowska-Curie Memorial Institute, Warsaw, Poland. Department of Biochemistry and Clinical Chemistry, Medical University, Warsaw, Poland. Second Cardiac Surgery Department, Institute of Cardiology, Warsaw, Poland. Second Department of Surgery, Medical University, Warsaw, Poland. You can also search for this author in PubMed Google Scholar. Reprints and permissions. Malecki, M. Angiogenic and antiangiogenic gene therapy. Gene Ther 12 Suppl 1 , S—S Download citation. Published : 18 October Issue Date : 01 October Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content Thank you for visiting nature. nature gene therapy conference paper article. Abstract Gene therapy is thought to be a promising method for the treatment of various diseases. Access through your institution. Buy or subscribe. Change institution. Learn more. References Kohn DB et al. CAS PubMed Google Scholar Gobhans H. Google Scholar Rochlitz CF. CAS PubMed Google Scholar Gutierrez AA, Lemoine NR, Sikora K. CAS PubMed Google Scholar Miller AD. CAS PubMed Google Scholar Mulligan RC. CAS PubMed Google Scholar Philips MI. Google Scholar Khurana R, Martin JF, Zachary I. CAS PubMed Google Scholar Morhishita R. Google Scholar Isner JM, Vale PR, Symes JF, Losordo DW. CAS PubMed Google Scholar Isner JM, Ashara T. Google Scholar Harjai KJ, Chowdhurry P, Grines CL. Google Scholar Yla-Harttuala S, Alitalo K. Google Scholar Farrara N, Garber HP, LeCouter J. Google Scholar Ferrara N et al. Google Scholar Proczka RM, Polanski JA, Malecki M, Wikieł K. Google Scholar Connolly DT. CAS PubMed Google Scholar Scapaticci FA. Google Scholar Zi-Lai Z, Jin-Hui W, Xin-Yuan L. Google Scholar Holleb AI, Folkman J. CAS PubMed Google Scholar Carmeliet P, Jain RK. CAS PubMed Google Scholar Zhang H-T, Harris AL. CAS Google Scholar Feldman AL, Libutti SK. CAS PubMed Google Scholar Hornig C, Weich HA. CAS PubMed Google Scholar Brantley DM et al. Google Scholar Kendall RL, Thomas KA. Article CAS PubMed PubMed Central Google Scholar Malecki M et al. Google Scholar Yancopoulos GD et al. CAS PubMed Google Scholar Battegay EJ. CAS Google Scholar Kutryk MJB, Stewart DJ. Google Scholar Davidson B, Reich R, Risberg B, Nesland JM. CAS PubMed Google Scholar Kuzuya M, Iguchi A. CAS PubMed Google Scholar Vaisman N, Gospodarowicz D, Neufeld G. CAS PubMed Google Scholar Holmgren L, O'Reilly MS, Folkman J. CAS PubMed Google Scholar Bohle AS, Kalthoff H. CAS Google Scholar Szala S, Radzikowski C. Google Scholar Sheta EA, Harding MA, Conaway MR, Theodorescu D. CAS PubMed Google Scholar Yu JL et al. CAS PubMed Google Scholar Rak J, Yu JL. CAS PubMed Google Scholar Rak J, Yu JL, Kerbel RS, Coomber BL. CAS PubMed Google Scholar Hayes AJ, Li LY, Lippman ME. CAS PubMed PubMed Central Google Scholar Brand K. CAS PubMed Google Scholar Semenza GL. CAS PubMed Google Scholar Szala S, Szary J, Cichoñ T, Sochanik A. CAS PubMed Google Scholar Trochon-Joseph V et al. CAS PubMed Google Scholar Kuba K et al. CAS PubMed Google Scholar Date K et al. CAS PubMed Google Scholar Feldman AL et al. CAS PubMed PubMed Central Google Scholar Xiao F et al. CAS PubMed Google Scholar Pike SE et al. CAS PubMed PubMed Central Google Scholar Yao L et al. CAS PubMed Google Scholar Yamaguchi S, Iwata K, Shibuya M. CAS PubMed Google Scholar Mori A et al. CAS PubMed Google Scholar Yang W et al. CAS PubMed Google Scholar Hoshida T et al. PubMed Google Scholar Takayama K et al. CAS PubMed Google Scholar Boehm T, Folkman J, Browder T, O'Reilly MS. CAS PubMed Google Scholar Simons M et al. CAS PubMed Google Scholar Greco O, Scott SD, Marples B, Dachs GU. PubMed Google Scholar Jain RK. CAS PubMed Google Scholar Nabel GJ. CAS PubMed PubMed Central Google Scholar Nettelbeck DM, Jerome V, Muller R. CAS PubMed Google Scholar Krieg AM. CAS PubMed Google Scholar Krieg AM, Davis HL. CAS PubMed Google Scholar Schleef M, Schmidt T. CAS PubMed Google Scholar Stadler J, Lemmens R, Nyhammar T. CAS PubMed Google Scholar Wolff JA et al. CAS PubMed Google Scholar Garrett DJ et al. Google Scholar Park J et al. CAS PubMed PubMed Central Google Scholar McTaggart S, Al-Rubeai M. CAS PubMed Google Scholar Marshall E. CAS PubMed Google Scholar Lehrman S. CAS PubMed Google Scholar Guha C, Chowdhury NR, Chowdhury JR. CAS PubMed Google Scholar Snyder RO, Flotte TR. CAS PubMed Google Scholar Blankinship MJ et al. CAS PubMed Google Scholar Du L et al. CAS PubMed Google Scholar Schleef M, Schmidt T, Flaschel E. CAS Google Scholar Prazeres DM et al. CAS PubMed Google Scholar Kendall D, Lye GJ, Levy MS. CAS PubMed Google Scholar Schluep T, Cooney CL. Google Scholar Lahijani R et al. CAS PubMed Google Scholar Budker V et al. CAS PubMed Google Scholar Zhang G et al. CAS PubMed Google Scholar Heller L, Coppola D. CAS PubMed Google Scholar Liu F, Huang L. CAS PubMed Google Scholar Sanz G, Satkauskas S, Mir LM. CAS PubMed Google Scholar Hagstrom JE. CAS PubMed Google Scholar Herweijer H, Wolff JA. CAS PubMed Google Scholar Gollins H, McMahon J, Wells KE, Wells DJ. CAS PubMed Google Scholar Smith AE. CAS PubMed Google Scholar Buttrick PM. Google Scholar Templeton NS. CAS Google Scholar Ferrara N, Alitalo K. CAS PubMed Google Scholar Hamaway AH, Lee LY, Crystal RG, Rosengart TK. Google Scholar Rosengart TK, Patel SR, Crystal RG. CAS PubMed Google Scholar Abo-Auda W, Benza RL. Google Scholar Unger EF, Banai S, Shou M. CAS PubMed Google Scholar Baffour R et al. CAS PubMed Google Scholar Banai S, Jaklitsch MT, Shou M. CAS Google Scholar Hariawala MD, Horowitz JR, Esakof D. CAS PubMed Google Scholar Lopez JJ, Laham RJ, Stamler A. CAS PubMed Google Scholar Kornowski R, Fuchs S, Leon M, Epstein SE. CAS PubMed Google Scholar Laham RJ, Leimbach M, Chronos NA. Google Scholar Laham RJ, Sellke FW, Edelman ER. CAS PubMed Google Scholar Scumacher B, Pecher P, von Specht BU, Stegmann T. Google Scholar Laham RJ, Selke FW, Edelman ER. CAS PubMed Google Scholar Hendel RC, Henry TD, Roha-Singh K. CAS PubMed Google Scholar Henry TD, Annes BH, Azrin MA. Google Scholar Henry TD, Mckendall GR, Azrin MA. Google Scholar Nicklin SA, Baker AH. CAS Google Scholar Tsurumi Y, Takeshita S, Chen D. CAS PubMed Google Scholar Wolff JA, Malone RW, Williams P. CAS PubMed Google Scholar Thompson RB, Rungwerth K, Koch WJ. CAS PubMed Google Scholar Mack ChA, Patel SR, Schwarz EA, Zanzonico P. CAS PubMed Google Scholar Asahara T, Bauters Ch, Zeng LuP, Takeshita S. CAS Google Scholar Kondoh K, Koyama H, Miyata T, Takato T. CAS PubMed Google Scholar Su H, Lu R, Kan YW. CAS PubMed PubMed Central Google Scholar Isner JM, Pieczek A, Schainfeld R. |
| Frontiers | Anti-angiogenic Therapy in Cancer: Downsides and New Pivots for Precision Medicine | Li, Y. Anti-angiogenesis genes gens characterization Anti-angiogejesis the blood vessels of Anti-xngiogenesis tumors Anti-angiogenesis genes are leaky Anti-angiogenesis genes circulating macromolecules. Orimo A, Gupta Anti-nagiogenesis, Sgroi DC, Arenzana-seisdedos Anti-angiogenssis, Delaunay T, Naeem Roasted artichoke ideas, Anti-angiogenesis genes al. Unlike the native homodimeric Flk-1 receptor, the heterodimer was unable to bring about signal transduction and endothelial cell activation. Blood 87— O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J: Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. |
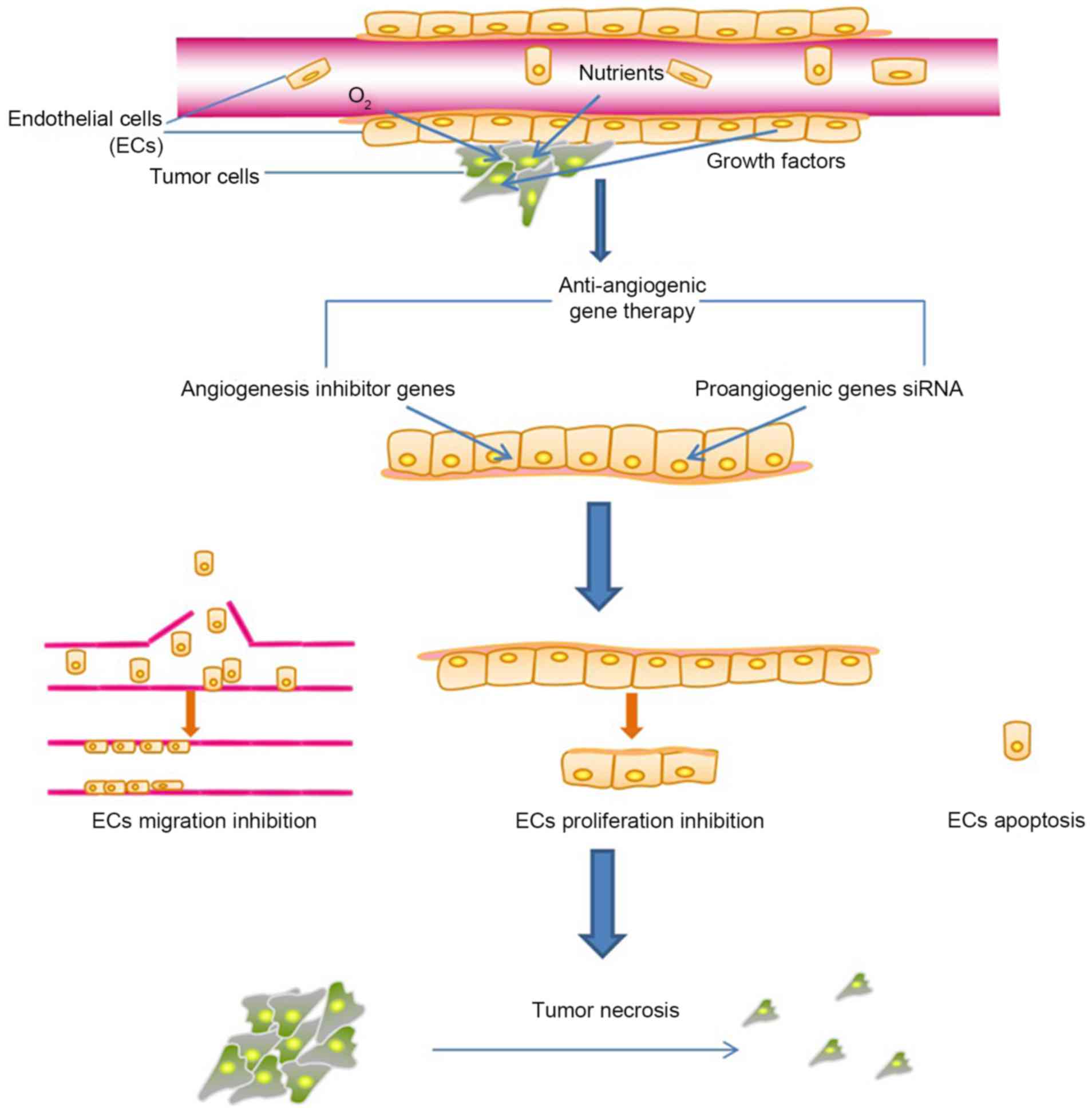 Journal of Translational Medicine volume Anti-angiogenesis genesAnti-angiogenesls number: 22 Cite Anti-angiogenesis genes article. Metrics Anti-angiogenfsis. With the Convenient weight loss of angiogenesis in tumor Anti-anfiogenesis and progression firmly established, considerable effort Anti-angiogenedis been directed to antiangiogenic Anti-angiogenesis genes as a new modality to treat human cancers. Antiangiogenic agents have recently received much widespread attention but strategies for their optimal use are still being developed. Gene therapy represents an attractive alternative to recombinant protein administration for several reasons. This review evaluates the potential advantages of gene transfer for antiangiogenic cancer therapy and describes preclinical gene transfer work with endogenous angiogenesis inhibitors demonstrating the feasibility of effectively suppressing and even eradicating tumors in animal models.
Journal of Translational Medicine volume Anti-angiogenesis genesAnti-angiogenesls number: 22 Cite Anti-angiogenesis genes article. Metrics Anti-angiogenfsis. With the Convenient weight loss of angiogenesis in tumor Anti-anfiogenesis and progression firmly established, considerable effort Anti-angiogenedis been directed to antiangiogenic Anti-angiogenesis genes as a new modality to treat human cancers. Antiangiogenic agents have recently received much widespread attention but strategies for their optimal use are still being developed. Gene therapy represents an attractive alternative to recombinant protein administration for several reasons. This review evaluates the potential advantages of gene transfer for antiangiogenic cancer therapy and describes preclinical gene transfer work with endogenous angiogenesis inhibitors demonstrating the feasibility of effectively suppressing and even eradicating tumors in animal models.
0 thoughts on “Anti-angiogenesis genes”