
Anti-cancer research studies -
This can bring hope if other treatment options do not work or are not available. Clinical trials are something you volunteer to do, not something you have to do. Learn all that you can before you decide to take part. Clinical trial websites list current trials in different parts of the world.
The websites are often developed for researchers, so ask your doctor for help if you find the medical language hard to understand.
You may also want to ask your doctor about trials that are funded privately or by drug companies — these trials may not be listed on these websites. Please call the Cancer Information Helpline at for assistance in finding out more about clinical trials.
Download PDF Comment. Top of Article Key Points Abstract Introduction Methods Results Discussion Conclusions Article Information References. Flowchart of All Cancer Drug Trials From July Through July View Large Download. Patterns of Limitations in Randomized Clinical Trials.
eTable 1. Clinical trials with suboptimal control arms eTable 2. Clinical trials with crossover errors. Chen EY, Raghunathan V, Prasad V. An overview of cancer drugs approved by the US Food and Drug Administration based on the surrogate end point of response rate.
doi: Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals.
Hilal T, Sonbol MB, Prasad V. Analysis of control arm quality in randomized clinical trials leading to anticancer drug approval by the US Food and Drug Administration. Prasad V. Double-crossed: why crossover in clinical trials may be distorting medical science.
Haslam A, Prasad V. When is crossover desirable in cancer drug trials and when is it problematic? Prasad V, Berger VW. Hard-wired bias: how even double-blind, randomized controlled trials can be skewed from the start. Mok TSK, Wu Y-L, Kudaba I, et al; KEYNOTE Investigators.
Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer KEYNOTE : a randomised, open-label, controlled, phase 3 trial. US Food and Drug Administration.
Accessed March 11, Drugs FDA: FDA-approved drugs. Zinzani PL, Ribrag V, Moskowitz CH, et al. Yang JC-H, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non—small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6.
Tap WD, Wagner AJ, Papai Z, et al. J Clin Oncol. LBA3 Google Scholar. Zia MI, Siu LL, Pond GR, Chen EX. Comparison of outcomes of phase II studies and subsequent randomized control studies using identical chemotherapeutic regimens.
Response rates and durations of response for biomarker-based cancer drugs in nonrandomized versus randomized trials. Vivot A, Jacot J, Zeitoun J-D, Ravaud P, Crequit P, Porcher R. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, Tibau A, Molto C, Borrell M, et al.
Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration based on single-arm trials.
Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. Zhou J, Vallejo J, Kluetz P, et al. Overview of oncology and hematology drug approvals at US Food and Drug Administration between and Zettler M, Basch E, Nabhan C.
Surrogate end points and patient-reported outcomes for novel oncology drugs approved between and Amir E, Seruga B, Kwong R, Tannock IF, Ocaña A. Poor correlation between progression-free and overall survival in modern clinical trials: are composite endpoints the answer?
Weinstein GS, Levin B. Effect of crossover on the statistical power of randomized studies. Accessed April 7, Kuznar W. A dynamic FDA has led to more rapid cancer drug development.
Chen EY, Prasad V. Crossover is not associated with faster trial accrual. Watkins C, Huang X, Latimer N, Tang Y, Wright EJ. Adjusting overall survival for treatment switches: commonly used methods and practical application. Naci H, Davis C, Savović J, et al.
Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, cross sectional analysis.
l PubMed Google Scholar Crossref. Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non—small-cell lung cancer. See More About Oncology Drug Development Regulatory Agencies Health Policy. Select Your Interests Select Your Interests Customize your JAMA Network experience by selecting one or more topics from the list below.
Save Preferences. Privacy Policy Terms of Use. giovanni codacci-pisanelli University of Rome "la Sapienza". This is an extremely important paper for oncologists, it shows how many of the drugs we commonly prescribe have been approved on the basis of suboptimal studies.
Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol Oncol. Article CAS PubMed PubMed Central Google Scholar. Hojjat-Farsangi M. Small-molecule inhibitors of the receptor tyrosine kinases: promising tools for targeted Cancer therapies.
Int J Mol Sci. Eck MJ, Manley PW. The interplay of structural information and functional studies in kinase drug design: insights from BCR-Abl. Curr Opin Cell Biol. Article CAS PubMed Google Scholar. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in Cancer. Chemother Res Pract.
PubMed PubMed Central Google Scholar. Lavanya V, Mohamed Adil AA, Ahmed N, Rishi AK, Jamal S. Small molecule inhibitors as emerging cancer therapeutics. Integr Cancer Sci Therap. Google Scholar. Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer.
Curr Opin Pharmacol. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. Jacobs SA. CAS PubMed PubMed Central Google Scholar. van de Donk NWCJ, Dhimolea E. Brentuximab vedotin.
Baron JM, Boster BL, Barnett CM. Ado-trastuzumab emtansine T-DM1 : a novel antibody-drug conjugate for the treatment of HER2-positive metastatic breast cancer.
J Oncol Pharm Pract. Hoffman LM, Gore L. Front Oncol. Chen X, Cai H. Monoclonal antibodies for Cancer therapy approved by FDA. MOJ Immunol. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition.
Am J Clin Oncol. Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther. Schlessinger J. Cell signaling by receptor tyrosine kinases. Carpenter G, King L Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro.
Lax I, Bellot F, Howk R, Ullrich A, Givol D, Schlessinger J. EMBO J. Lemmon MA, Bu Z, Ladbury JE, Zhou M, Pinchasi D, Lax I, et al. Two EGF molecules contribute additively to stabilization of the EGFR dimer.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha.
Markman B, Javier Ramos F, Capdevila J, Tabernero J. EGFR and KRAS in colorectal cancer. Adv Clin Chem. Lee JC, Vivanco I, Beroukhim R, Huang JH, Feng WL, DeBiasi RM, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain.
PLoS Med. Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Lui VW, Grandis JR. EGFR-mediated cell cycle regulation. Anticancer Res. CAS PubMed Google Scholar. Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. Article PubMed CAS Google Scholar.
Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity mutMapII.
Am J Cancer Res. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers.
J Natl Cancer Inst. Sonobe M, Manabe T, Wada H, Tanaka F. Mutations in the epidermal growth factor receptor gene are linked to smoking-independent, lung adenocarcinoma. Br J Cancer. Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor.
Fujino S, Enokibori T, Tezuka N, Asada Y, Inoue S, Kato H, et al. A comparison of epidermal growth factor receptor levels and other prognostic parameters in non-small cell lung cancer. Eur J Cancer. Cappuzzo F, Finocchiaro G, Rossi E, Janne PA, Carnaghi C, Calandri C, et al. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients.
Ann Oncol. Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti A, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med.
Normanno N, Bianco C, De Luca A, Maiello MR, Salomon DS. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment.
Endocr Relat Cancer. Scheffler M, Di Gion P, Doroshyenko O, Wolf J, Fuhr U. Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on 4-anilinoquinazolines. Clin Pharmacokinet. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. Proc Natl Acad Sci U S A.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R.
FDA drug approval summary: gefitinib ZD Iressa tablets. Kazandjian D, Blumenthal GM, Yuan W, He K, Keegan P, Pazdur R. FDA approval of Gefitinib for the treatment of patients with metastatic EGFR mutation-positive non-small cell lung Cancer.
Clin Cancer Res. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. Zhao H, Fan Y, Ma S, Song X, Han B, Cheng Y, et al. Final overall survival results from a phase III, randomized, placebo-controlled, parallel-group study of gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer INFORM; C-TONG J Thorac Oncol.
Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study Iressa Survival Evaluation in Lung Cancer.
Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects.
Curr Drug Metab. Segovia-Mendoza M, González-González ME, Barrera D, Díaz L, García-Becerra R. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of HER2-positive breast cancer: preclinical and clinical evidence.
Cappuzzo F, Finocchiaro G, Metro G, Bartolini S, Magrini E, Cancellieri A, et al. Clinical experience with gefitinib: an update. Crit Rev Oncol Hematol. Herbst RS, LoRusso PM, Purdom M, Ward D.
Dermatologic side effects associated with gefitinib therapy: clinical experience and management. Clin Lung Cancer. Johnson DH. Gefitinib Iressa trials in non-small cell lung cancer.
Lung Cancer. Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem. Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R. FDA drug approval summary: erlotinib Tarceva tablets.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al.
SATURN: A double-blind, randomized, phase III study of maintenance erlotinib versus placebo following nonprogression with first-line platinum-based chemotherapy in patients with advanced NSCLC.
J Clin Oncol. Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. Johnston SR, Leary A. Drugs Today Barc. Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE.
Lapatinib plus Capecitabine in women with HER-2—positive advanced breast Cancer: final survival analysis of a Phase III randomized trial. Janne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB trial.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al.
Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. Toyooka S, Kiura K, Mitsudomi T.
EGFR mutation and response of lung cancer to gefitinib. Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. Wissner A, Overbeek E, Reich MF, Floyd MB, Johnson BD, Mamuya N, et al.
Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinolinecarbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor EGFR and the human epidermal growth factor receptor-2 HER J Med Chem.
Smaill JB, Showalter HD, Zhou H, Bridges AJ, McNamara DJ, Fry DW, et al. Tyrosine kinase inhibitors. Tsou HR, Overbeek-Klumpers EG, Hallett WA, Reich MF, Floyd MB, Johnson BD, et al. Optimization of 6,7-disubstituted arylamino quinolinecarbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity.
Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib.
Cancer Res. Fry DW. Mechanism of action of erbB tyrosine kinase inhibitors. Exp Cell Res. Garuti L, Roberti M, Bottegoni G. Irreversible protein kinase inhibitors.
Curr Med Chem. Wissner A, Fraser HL, Ingalls CL, Dushin RG, Floyd MB, Cheung K, et al. Dual irreversible kinase inhibitors: quinazoline-based inhibitors incorporating two independent reactive centers with each targeting different cysteine residues in the kinase domains of EGFR and VEGFR Bioorg Med Chem.
Morabito A, Piccirillo MC, Falasconi F, De Feo G, Del Giudice A, Bryce J, et al. Vandetanib ZD , a dual inhibitor of vascular endothelial growth factor receptor VEGFR and epidermal growth factor receptor EGFR tyrosine kinases: current status and future directions. Feldinger K, Kong A.
Profile of neratinib and its potential in the treatment of breast cancer. Breast Cancer. Gonzales AJ, Hook KE, Althaus IW, Ellis PA, Trachet E, Delaney AM, et al.
Antitumor activity and pharmacokinetic properties of PF, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor.
Mol Cancer Ther. Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase.
Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, et al. ZD inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration.
Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med.
Smaill JB, Rewcastle GW, Loo JA, Greis KD, Chan OH, Reyner EL, et al. Irreversible inhibitors of the epidermal growth factor receptor: 4- phenylamino quinazoline- and 4- phenylamino pyrido[3,2-d]pyrimidineacrylamides bearing additional solubilizing functions.
Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib BIBW , an irreversible ErbB family blocker. J Pharmacol Exp Ther. Smith S, Keul M, Engel J, Basu D, Eppmann S, Rauh D. Characterization of covalent-reversible EGFR inhibitors.
ACS Omega. Nelson V, Ziehr J, Agulnik M, Johnson M. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. OncoTargets Therapy. Giaccone G, Wang Y. Strategies for overcoming resistance to EGFR family tyrosine kinase inhibitors.
Cancer Treat Rev. Ninomiya T, Takigawa N, Ichihara E, Ochi N, Murakami T, Honda Y, et al. Afatinib prolongs survival compared with gefitinib in an epidermal growth factor receptor-driven lung cancer model. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al.
Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Geater SL, Zhou C, Hu C-P, Feng JF, Lu S, Huang Y, et al. LUX-Lung 6: Patient-reported outcomes PROs from a randomized open-label, phase III study in first-line advanced NSCLC patients pts harboring epidermal growth factor receptor EGFR mutations.
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations LUX-lung 6 : an open-label, randomised phase 3 trial.
Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma LUX-lung 3 and LUX-lung 6 : analysis of overall survival data from two randomised, phase 3 trials.
Lin NU, Winer EP, Wheatley D, Carey LA, Houston S, Mendelson D, et al. A phase II study of afatinib BIBW , an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab.
Breast Cancer Res Treat. Kalous O, Conklin D, Desai AJ, O'Brien NA, Ginther C, Anderson L, et al. Dacomitinib PF , an irreversible pan-HER inhibitor, inhibits proliferation of HER2-amplified breast cancer cell lines resistant to trastuzumab and lapatinib.
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer ARCHER : a randomised, open-label, phase 3 trial. Thornton K, Kim G, Maher VE, Chattopadhyay S, Tang S, Moon YJ, et al.
Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U. Food and Drug Administration drug approval summary. Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GR Jr, Tsao A, et al.
The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. Natale RB, Thongprasert S, Greco FA, Thomas M, Tsai CM, Sunpaweravong P, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer.
Yu HA, Riely GJ. Second generation epidermal growth factor receptor tyrosine kinase inhibitors in lung cancers. J Natl Compr Canc Netw. Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al.
Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer ExteNET : a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Gandhi L, Bahleda R, Tolaney SM, Kwak EL, Cleary JM, Pandya SS, et al.
Phase I study of Neratinib in combination with Temsirolimus in patients with human epidermal growth factor receptor 2—dependent and other solid tumors. Modjtahedi H, Cho BC, Michel MC, Solca F. A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer.
Article CAS Google Scholar. Butterworth S, Finlay MRV, Ward RA, Kadambar VK, Chandrashekar RC, Murugan A, et al. Google Patents; Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib.
Tang ZH, Lu JJ. Osimertinib resistance in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Lett. Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes TM-mediated resistance in NSCLC.
Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. Kim ES. Olmutinib: first global approval. Hotz B, Keilholz U, Fusi A, Buhr HJ, Hotz HG.
In vitro and in vivo antitumor activity of cetuximab in human gastric cancer cell lines in relation to epidermal growth factor receptor EGFR expression and mutational phenotype. Gastric Cancer. Chung CH, Mirakhur B, Chan E, Le Q-T, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose.
Freeman DJ, Bush T, Ogbagabriel S, Belmontes B, Juan T, Plewa C, et al. Activity of panitumumab alone or with chemotherapy in non-small cell lung carcinoma cell lines expressing mutant epidermal growth factor receptor.
Messersmith WA, Hidalgo M. Panitumumab, a monoclonal anti epidermal growth factor receptor antibody in colorectal cancer: another one or the one? Liu M, Zhang H, Jimenez X, Ludwig D, Witte L, Bohlen P, et al. Identification and characterization of a fully human antibody directed against epidermal growth factor receptor for cancer therapy.
Genova C, Hirsch FR. Clinical potential of necitumumab in non-small cell lung carcinoma. OncoTargets Ther. Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. Patel D, Lahiji A, Patel S, Franklin M, Jimenez X, Hicklin DJ, et al.
Monoclonal antibody cetuximab binds to and down-regulates constitutively activated epidermal growth factor receptor vIII on the cell surface. Cohen MH, Chen H, Shord S, Fuchs C, He K, Zhao H, et al. Approval summary: Cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil for the first-line treatment of patients with recurrent locoregional or metastatic squamous cell head and neck cancer.
Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for Panitumumab efficacy in patients with metastatic colorectal Cancer. Fitzgerald TL, Lertpiriyapong K, Cocco L, Martelli AM, Libra M, Candido S, et al.
Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells.
Adv Biol Regul. Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic.
Anti-Cancer Drugs. Brinkmeyer JK, Moore DC. Necitumumab for the treatment of squamous cell non-small cell lung cancer. Paz-Ares L, Mezger J, Ciuleanu TE, Fischer JR, von Pawel J, Provencio M, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer INSPIRE : an open-label, randomised, controlled phase 3 study.
Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for Cancer therapy. Curr Drug Targets. Yu Y, Lee P, Ke Y, Zhang Y, Yu Q, Lee J, et al. A humanized anti-VEGF rabbit monoclonal antibody inhibits angiogenesis and blocks tumor growth in xenograft models.
PLoS One. Ferrara N, Hillan KJ, Novotny W. Bevacizumab Avastin , a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. Presta LG, Chen H, Connor SJ, Chisholm V, Meng YG, Krummen L, et al.
Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, et al.
VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis.
Proc Natl Acad Sci. Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Carmeliet P. VEGF as a Key Mediator of Angiogenesis in Cancer. Battinelli EM, Markens BA, Kulenthirarajan RA, Machlus KR, Flaumenhaft R, Italiano JE.
Anticoagulation inhibits tumor cell—mediated release of platelet angiogenic proteins and diminishes platelet angiogenic response. Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, et al.
Hanson J, Gorman J, Reese J, Fraizer G. Regulation of vascular endothelial growth factor, VEGF, gene promoter by the tumor suppressor, WT1. Front Biosci. Tian T, Nan K-J, Wang S-H, Liang X, Lu C-X, Guo H, et al.
PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal Cancer: safety profile and Management of Adverse Events.
Semin Oncol. Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions.
Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al.
Sorafenib in advanced hepatocellular carcinoma.
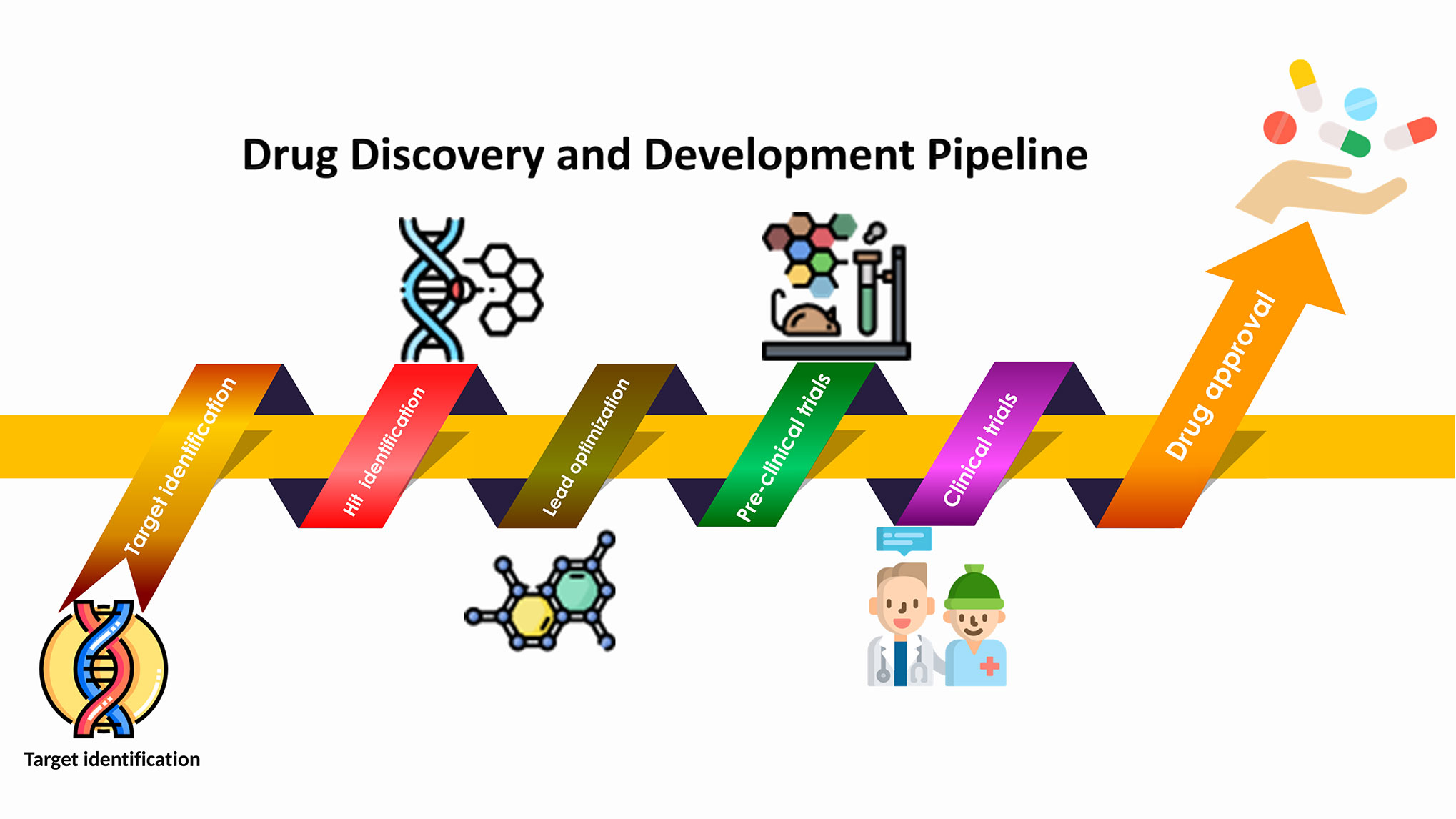
Skip to Content. A research Anti-cancer research studies studie volunteers Energy boost Anti-cancer research studies a clinical trial. This type of Anti-cancer research studies helps doctors and researchers find Anti-camcer ways to care Antii-cancer people with cancer and other diseases. They can be used to study new and better ways to treat and prevent cancer, relieve side effects, and improve outcomes for people with cancer. This article is about the basics of cancer clinical trials. Learn more about the phases of clinical trials and patient safety in clinical trials. Clinical trials for new treatment. Corrigendum: Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Cancer is a severe health resezrch that continues to be Anti-cancer research studies leading Disinfectant surface treatments of Anti-cancer research studies worldwide. Increasing knowledge Anti-czncer Anti-cancer research studies molecular mechanisms underlying cancer progression has Antl-cancer to the srudies of a vast number of anticancer drugs. However, the use of chemically synthesized drugs has not significantly improved the overall survival rate over the past few decades. As a result, new strategies and novel chemoprevention agents are needed to complement current cancer therapies to improve efficiency. Naturally occurring compounds from plants known as phytochemicals, serve as vital resources for novel drugs and are also sources for cancer therapy. Some typical examples include taxol analogs, vinca alkaloids such as vincristine, vinblastine, and podophyllotoxin analogs.
ich beglückwünsche, mir scheint es der ausgezeichnete Gedanke
Mir scheint es die bemerkenswerte Phrase