
Snake venom neutralizer -
In the U. Worldwide, an estimated 4. And the antidote only halts the damage inflicted by a small number of species.
Ours seems to show broad-spectrum ability to stop cell destruction across species on many continents, and that is quite a big deal," said doctoral student Jeffrey O'Brien, lead author of a recent study published in the Journal of the American Chemical Society.
Zeroing in on protein families common to many serpents, the UCI researchers demonstrated that they could halt the worst effects of cobras and kraits in Asia and Africa, as well as pit vipers in North America.
The team synthesized a polymer nanogel material that binds to several key protein toxins, keeping them from bursting cell membranes and causing widespread destruction. O'Brien knew he was onto something when the human serum in his test tubes stayed clear, rather than turning scarlet from venom's typical deadly rupture of red blood cells.
Chemistry professor Ken Shea, senior author of the paper, explained that the venom -- a "complex toxic cocktail" evolved over millennia to stay ahead of prey's own adaptive strategies -- is absorbed onto the surface of nanoparticles in the new material and is permanently sequestered there, "diverted from doing harm.
Thanks to the use of readily available, nonpoisonous components, the "nanodote" has a long shelf life and costs far less. The existing antidote is made by injecting horses with venom, waiting weeks for the animals to develop antibodies, then extracting their blood and shipping it from Mexico or Australia to places that can afford it.
The process is not allowed in the U. Major suppliers have discontinued shipments to many markets. In contrast, "our treatment costs pennies on the dollar and, unlike the current one, requires no refrigeration," O'Brien said. Since publishing their findings, the researchers have discovered that scorpion and spider bite infections may also be slowed or stopped via their invention.
They have patents pending and are seeking public and private funding to move forward with clinical trials and product development. Additionally, Shea's group pioneered a synthetic antidote for bee melittin -- the ingredient in stings that can kill people who have an allergic reaction -- using similar methods.
We have more work to do, and this is why we're seeking a fairly significant infusion of resources. The U. Department of Defense's research arm financed the first phase of the laboratory work. They need something they can use in the field to at least delay the spread of the venom.
Materials provided by University of California - Irvine. Note: Content may be edited for style and length. Science News. Facebook Twitter Pinterest LinkedIN Email. Popular Mechanics. Archived from the original on Archived from the original on 13 October Poison Center Tampa. Archived from the original on 1 April National Institutes of Health.
Archived from the original on 30 March The Dangerous Snakes of Africa. Ralph Curtis Books. Dubai: Oriental Press. Regional Office for South-East Asia, World Health Organization Guidelines for the management of snakebites 2nd ed.
World Health Organization Snakebite envenoming: a strategy for prevention and control. Portal : Medicine. Categories : Antitoxins Toxicology treatments.
Hidden categories: Articles with short description Short description matches Wikidata Chemicals that do not have a ChemSpider ID assigned Infobox drug articles without a structure image Chemical articles without CAS registry number Articles without EBI source Chemical pages without DrugBank identifier Articles without KEGG source Articles without InChI source Articles without UNII source Drugs missing an ATC code Drugs with no legal status Articles containing unverified chemical infoboxes Articles containing potentially dated statements from All articles containing potentially dated statements All articles with unsourced statements Articles with unsourced statements from October Wikipedia articles needing clarification from October Articles containing potentially dated statements from Toggle limited content width.
Snake antivenin, snake antivenene, snake venom antiserum, antivenom immunoglobulin. Most are harmless, but others have toxic saliva and at least five species, including the boomslang Dispholidus typus , have caused human fatalities. Sea snakes , Taipans , Brown snakes , Coral snakes , Kraits , King Cobra , Mambas , Cobras.
True vipers and pit vipers , including rattlesnakes and copperheads and cottonmouths. South American Rattlesnake Crotalus durissus and fer-de-lance Bothrops asper.
Saw-scaled Viper Echis carinatus , Russell's Viper Daboia russelli , Spectacled Cobra Naja naja , Common Krait Bungarus caeruleus. Australian copperheads , Tiger snakes , Pseudechis spp.
Polyvalent crotalid antivenin CroFab - Crotalidae Polyvalent Immune Fab Ovine. North American pit vipers all rattlesnakes , copperheads , and cottonmouths. Pit vipers and rattlesnakes. Mambas , Cobras , Rinkhalses , Puff adders Unsuitable small adders: B.
worthingtoni , B. atropos , B. caudalis , B. cornuta , B. heraldica , B. Australia is the only country in the world that has snake venom detection kits. They consist of a rapid two step enzyme immunoassay in which wells are coated with antibodies to the various snake venoms.
A swab from the bite site, blood, or urine helps to select the type of snake antivenom which may have to be used. Note that the primary purpose of the venom detection kit is not to decide whether envenomation has occurred i.
whether antivenom is indicated , but to help to choose the appropriate antivenom if required. Skip to main content. School of Biomedical Sciences Our Departments and Centres Biochemistry and Pharmacology Engage AVRU Discover What is antivenom? WHAT IS ANTIVENOM?
HOW IS ANTIVENOM MADE? Horse blood inoculated with tiger and brown snake venoms When injected into a patient, the binding sites on the antibody fragments bind to the venoms or venom components in the circulation and neutralize the activity of the venoms in the patient.
Australian Snake Antivenoms Produced in: Other Australian Antivenoms Produced in: Tiger snake antivenom Horses Funnel web spider antivenom Rabbits Brown snake antivenom Horses Redback spider antivenom Horses Taipan antivenom Horses Australian paralysis tick antivenom Dogs Black snake antivenom Horses Box jellyfish antivenom Sheep Death adder antivenom Horses Stonefish antivenom Horses Sea snake antivenom Horses Polyvalent snake antivenom Horses.
Australian Snake Antivenoms. Other Australian Antivenoms.
Currently, the use of antivenoms is the only available treatment neutalizer envenomation caused vvenom venomous animals namely, snake, scorpion, spider, tick nneutralizer jelly fish. Snake venom neutralizer Circadian rhythm research generally produced in Performance-enhancing cooking oils animals, mostly in Snake venom neutralizer. A large percentage of the population is allergic to horse proteins. Several animals are known to be resistant to snakebites and the antihemorrhagic and anti-lethal components have been isolated from sera of opossum, mongoose, meerkat and hedgehog, as well as from venomous and non-venomous snakes. Anti-lethal factor named Lethal Toxin Neutralizing Factor LTNF has been isolated in purity from opossum Didelphis virginiana serum by high pressure liquid chromatography HPLC.Snake venom neutralizer -
Pre-incubation of rutin and RS with fibrinogen Figures 6A,B or albumin Figures 6C,D decreased the fluorescence of their Trp residues, indicating a possible alteration of their 3D structure, which was not observed by pre-incubation with SA.
FIGURE 6. Activity of rutin, RS and SA at different concentrations on the fluorescence spectra of A,B bovine fibrinogen and C,D BSA. Data are expressed as RFU, and assays were carried out in triplicate. As the focus of this work is the use of rutin and RS as therapeutic compounds for the treatment of snakebites, the possible modulation of BjV biological activities by rutin and RS was first assessed in vitro.
Secondly, the direct activity of rutin, RS and SA was also tested in jararhagin — the most abundant SVMP in BjV Sugiki et al. As observed for fibrinogen, albumin and PDI, rutin and RS decreased the fluorescence of Trp residues in BjV proteins Figure 7A and jararhagin Figure 7B.
However, SA only modulated the fluorescence of jararhagin. In order to verify whether protein conformational alterations were also modifying BjV activities, in vitro tests were undertaken. FIGURE 7. Activity of rutin, RS and SA on the fluorescence spectra of A BjV and B jararhagin.
Data were expressed as RFU. Activity of rutin, RS, SA, AEBSF SVSP inhibitor and o-phe SVMP inhibitor on enzymatic activities of BjV protein families in vitro : C LAAO, D hyaluronidase, E SVSP and F SVMP. SA and o-phe were tested only for SVMP activity, and AEBSF was tested only for SVSP activity.
Rutin and RS minimally inhibited LAAO activity max. of However, differently from rutin, RS inhibited BjV hyaluronidase activity Figure 7D in a concentration-dependent manner, showing a maximum of LAAO and hyaluronidase are minor parts of BjV, and therefore, other major protein families of BjV were chosen to be tested, as SVSP and SVMP.
SVSP activity Figure 7E was completely inhibited On the other hand, rutin did not alter the collagenolytic activity of SVMP Figure 7F , while BjV pre-incubated with o-phe, an SVMP inhibitor totally blocked this activity.
To confirm RS and SA ability to inhibit SVMP, other SVMP activities were tested. The proteolytic activity of BjV was analyzed on the substrates gelatin, casein and bovine fibrinogen Figures 8 — 10 , lanes 2. BjV alone cleaved protein bands of 75 kDa— kDa bands for gelatin, 25 kDa—30 kDa bands for casein α-casein and 60 kDa bands for fibrinogen α-chain , and subsequent increases in lower molecular weight bands, related to products of protein degradation of substrates.
BjV pre-incubated with AEBSF Figures 8A — 10A,E or rutin Figures 8A — 10A , B , lanes 3—7 minimally attenuated proteolysis maximum of As observed for the collagenolytic activity, SA exhibited lower rates of proteolysis inhibition than RS Figures 8A — 10A,D , lanes 3—7 , and maximum decreases of In addition, RS and SA decreased the degradation of fibrinogen by jararhagin and the degradation of gelatin by the collagenase from C.
histolyticum , indicating a broader modulation of different agents and toxins. FIGURE 8. A—D Activity of rutin, RS and SA at different concentration on BjV-induced gelatinolytic activity in vitro. Gelatin incubated without BjV lanes 1 or with BjV solutions at 1. Arrows refer to interest bands of 75 kDa— kDa, relative to non-degraded gelatin.
FIGURE 9. A—D Activity of rutin, RS and SA at different concentration on BjV-induced caseinolytic activity in vitro. Casein incubated without BjV lanes 1 or with BjV solutions at 1.
Arrows refer to interest bands of 25 kDa—30 kDa approximately , relative to non-degraded α-casein. FIGURE A—D Activity of rutin, RS and SA at different concentration on BjV-induced fibrinogenolytic activity in vitro.
Fibrinogen incubated without BjV lanes 1 or with BjV solutions at 1. Arrows refers to interest bands of 60 kDa approximately , relative to non-degraded fibrinogen α-chain.
Besides the proteolysis of proteins, SVMP also showed clotting activity due to the activation of coagulation factors, as factor X and prothrombin Sano-Martins et al.
Since the in vitro clotting activity is a well-established characteristic of BjV, this property was tested for rutin, RS or SA using bovine fibrinogen Figure 11B or mouse plasma Figure 11A as substrates. The clotting time of fibrinogen or mouse plasma was minimally decreased by rutin 5.
Differently, RS decreased BjV clotting activity in a concentration-dependent manner, ranging from Activity of rutin, RS or AS at different concentrations on BjV activities in vitro : A clotting of mouse plasma or B bovine fibrinogen.
Data were expressed as clotting activity in seconds after the addition of BjV 0. C Prothrombin activation. Data were expressed as percentage of activity, and assays were carried out in triplicates.
Therefore, rutin minimally interfered with BjV activities in vitro. Nonetheless , pre-incubation of RS or SA with BjV inhibited important activities thereof, mainly those related to SVMP activity. In addition, RS also displayed broader effects related to a direct modulation of coagulation, evidencing its potential as an anticoagulant compound.
Since rutin showed potential beneficial effects on envenomation in vivo Sachetto et al. For in vivo experiments, rutin and RS were tested in concentrations 9 times higher than those used for BjV, and therefore, at this dosage, rutin pre-incubated with BjV did not alter its proteins, while pre-incubation with RS or SA inhibited partially the SVMP biological activities.
To analyze mouse survival as well as hemostatic disturbance, BjV was intraperitoneally injected in mice. Two different doses of BjV were used: 2×LD 50 was used to simulate moderate envenomation and evaluate hemostatic parameters, whereas 3×LD 50 was used to evaluate severe envenomation.
Both rutin and RS were preincubated with BjV, and tested regarding its protective potential in vivo. The moderate envenomation model did not induce mortality in mice in 48 h. BjV also evokes other hemostatic disturbances in vivo , as thrombocytopenia and hypofibrinogenemia.
In addition to the hemostatic disturbances described above, BjV also evoked hemorrhages in the abdominal wall of all envenomed mice. However, the pre-incubation of BjV with RS or o-phe completely prevented it.
Data are expressed as mean ± SEM. The severe envenomation model allowed the observation of mice survival for 48 h after 3×LD 50 BjV injection Figure Animals injected with BjV and bacitracin A a thiol isomerase inhibitor showed even lower survival rates The results indicated that rutin, RS, AS and o-phe inhibited the toxic activity of BjV, preserving mice survival in a severe model of systemic envenomation.
Furthermore, the thiol isomerase inhibition by bacitracin A reduced mice survival, which indicate a protective role of these enzymes in the organism during envenomation. After 48 h of venom administration, hemostatic parameters of surviving mice were analyzed. The analysis of blood smears revealed that envenomed mice presented morphological alterations as the presence of anisocytosis, polychromasia, schizocytes, dacryocytes and acanthocytes.
This indicated that SVMP inhibition and rutin did not prevent RBC drop in the severe envenomation. However, RS and SA could prevent it, indicating the effectiveness of succinate groups on this parameter, regardless of SVMP inhibition.
In one mouse that received BjV alone and died before 48 h, organs were collected for histological analyses. Skeletal muscles in the abdominal wall, at the face that had peritoneum intermediating their direct contact with BjV, showed paler eosinophilic coloration in their cytoplasm, implying that they had been damaged.
Areas of hemorrhage were also observed at this face, but inflammatory infiltrate was not extensive. Muscle fibers present in the diaphragm showed the same lesions.
In mice that received BjV preincubated with rutin and that survived for 48 h, a decreased extension of muscle fibers, in the abdominal wall and diaphragm showed decreased coloration of cytoplasm, and hemorrhage was less intense.
In the mouse that received BjV preincubated with RS, no changes were observed. Thereby, the results of in vivo envenomation showed that BjV induced systemic hematological and hemostatic alterations and evoked animal lethality.
SVMP inhibition by o-phe and SA prevented the hemorrhagic and lethal activity of BjV, but did not show effects on other parameters.
Whereas rutin induced a partial improvement of fibrinogen levels, RS improved hematological and hemostatic parameters and completely inhibited BjV-induced hemorrhagic activity. This indicates that RS effectiveness is not limited to its succinate moieties nor SVMP inhibitory activity.
Moreover, rutin and RS ensured mice survival, showing their potential to neutralize the venom toxic activities, preventing the lethality evoked by BjV.
BjV is composed by a complex mixture of biomolecules, mostly proteins, as SVMP, SVSP, LAAO, hyaluronidases, among others Koh et al. These proteins present in vitro and in vivo activities, both in experimental models as well as in bitten patients, inducing hemostatic disturbances, bleeding and inflammatory reactions Sano-Martins et al.
The severity of Bothrops envenomation is stratified as mild, moderate or severe, for proper patient treatment and antivenom administration França et al. jararaca snakebite envenomation induces systemic alterations, as thrombocytopenia, a decrease in RBC counts and hypofibrinogenemia, both in animal models Yamashita et al.
Thus, the analysis of hemostatic disturbances, such as blood incoagulability, hypofibrinogenemia, and other hematological alterations are relevant parameters to evaluate both the severity of envenomation and the effectiveness of antivenom administration Santoro et al.
Therefore, the possible inhibitory potential of rutin and RS were tested regarding the characteristic enzymatic activities of BjV in vitro , as well as their potential to inhibit the toxicity and lethality of BjV in experimental envenomation models.
In order to achieve that, RS was synthetized. It showed higher water-solubility than rutin, in agreement with previous findings that reported an increase in 80 times in its solubility Pedriali et al. Furthermore, the differences between rutin and RS by HPLC and LC-MSE analyses were related to the substitutions of hydroxyl groups in the sugar moieties of rutin for succinate groups, as shown previously Pedriali, , which indicated the effectiveness of the rutin succinylation process.
Rutin succinylation did not alter the characteristic activities of rutin, however the observed difference between rutin and RS antioxidant capacity may be due to the normal range of antioxidant activity of the flavonoids, which differs even within quercetin molecules. Besides, the results are in accordance with previous reports in which it was shown that RS has a lower antioxidant activity due to its lack of stabilization of carboxyl groups by hydroxyl groups in RS Pedriali, ; Pedriali et al.
Differently from rutin and RS, succinic acid presented a calcium quenching activity, which may be related to its ability of interacting and forming complexes with metal ions Domingo et al.
Another key feature of rutin is its ability to inhibit PDI. It is known that PDI is susceptible to alterations of structure and function depending on the redox state and its interaction with substrates Bekendam et al.
PDI possesses 5 Trp residues, and it was already observed that GSSG — a known substrate that interacts with PDI Raturi and Mutus, — induces a decrease in fluorescence of Trp residues in PDI Ado et al.
Extracellular PDI is important for thrombus formation in vivo and rutin inhibits PDI both in vitro and in vivo. Rutin and analogous components inhibit thrombus formation in mice, as evaluated by the decrease in platelet accumulation and fibrin deposition Jasuja et al. In addition, rutin was already shown to have the potential of interfering with other proteins in the blood, as showed by the results for BSA and fibrinogen.
The activity of rutin on BSA was demonstrated earlier, and the decrease in albumin fluorescence was related with rutin binding by means of hydrogen bridges and weak van der Waals forces Sengupta et al. The decrease in Trp residue fluorescence is also associated with conformational alterations of fibrinogen, since the Trp residues are mostly located in the hydrophobic portion of fibrinogen Zhang et al.
Our results confirm the interaction of rutin and RS with BSA and fibrinogen, which is important to understand their role in vivo , since BSA is relevant for the transport and bioavailability of exogenous components in the organism, whereas fibrinogen is a key component of hemostasis.
Regarding hemostasis components, SVSP and SVMP — the major protein families found in BjV — are both responsible for the clotting activity of BjV Santoro and Sano-Martins, ; Yamashita et al. SVMP act as procoagulant proteins due to its ability to directly activate factor X and prothrombin Antunes et al.
SVSP may act as thrombin-like enzymes, which are capable of inducing in vitro and in vivo coagulation Santoro and Sano-Martins, ; Sano-Martins et al. Quercetins reversely inhibit serine proteases, as human thrombin and, possibly, thrombin-like enzymes Mozzicafreddo et al.
This effect is concentration-dependent, as observed for the inhibition of SVSP activity by rutin. Moreover, the different structures of flavonoids interfere on their potential to bind to serine proteases Mozzicafreddo et al.
Rutin was previously studied when pre-incubated with BjV, and it did not show significant inhibitory activity on BjV activities in vitro Sachetto et al.
However, the reduction in fluorescence of Trp residues has been considered an indicative of conformational alterations of proteins present in snake venoms Hashkel et al.
In fact, the modest inhibition of BjV toxins by rutin may be due to its indirect activities, as an antioxidant that neutralizes H 2 O 2 generation by LAAO in vitro , or due to other effects that occurred only when high concentrations of rutin, as for SVSP inhibition, were used.
However, RS displayed a higher ability to inhibit BjV toxins in vitro , as hyaluronidases and SVMP. Snake venom hyaluronidases are often related to the degradation of extracellular matrix components, mainly hyaluronic acid, contributing to local damage and venom propagation in vivo. Flavonoids are hyaluronidase inhibitors Bala et al.
The SVMP protein family is the most abundant in BjV and show several activities in vitro and in vivo Markland and Swenson, As demonstrated previously Sachetto et al.
Degradation of protein components is an important activity of SVMP, mainly related to the hydrolysis of basal membrane components of capillary vessels, which is a fundamental step for the development of hemorrhages induced by venom in vivo Gutiérrez et al. Studies have demonstrated the relevance of SVMP on systemic envenomation caused by B.
asper , and that SVMP inhibitors decreased the clotting activity of venom in vitro, and defibrinogenation and hemorrhagic activities in vivo Rucavado et al. As showed herein, RS effectively inhibited both hemorrhagic and defibrinogenating activities of BjV on the moderate model of envenomation.
However, these inhibitors only partially prevented the increase in vascular permeability and mortality of envenomed animals Rucavado et al. The lethality of Viperidae snake venoms is attributed to intravascular consumption of coagulation factors induced by procoagulating venoms, and to blood leakage that leads to cardiovascular collapse by hemorrhagic venoms Gutierrez et al.
Studies suggest that Bothrops venoms lethality is multifactorial Maria et al. Our results demonstrate that BjV SVMP inhibition by RS, SA or o-phe is effective on preventing the hemorrhages and lethality induced by BjV. However, RS effects were certainly broader, and not only due to SVMP inhibition, as demonstrated by the beneficial effect of RS on blood cell counts during envenomation.
The decrease in RBC parameters by Bothrops envenomation has been already related to the occurrence of local and systemic bleedings and microangiopathic hemolytic anemia in animals Senise et al. Accordingly, our results showed that mice with lower RBC counts also manifested morphologic alterations of RBC — indicating that venom-induced intravascular hemolysis occurred Senise et al.
The precise mechanisms of thrombocytopenia in B. jararaca envenomation are not completely elucidated yet, although it is known that it is not related to SVMP, SVSP or local hemorrhagic injury Yamashita et al. Furthermore, more severely envenomed patients manifested a more prominent platelet decrease Santoro et al.
As mentioned above, blood incoagulability is attributed to SVMP and SVSP activities Yamashita et al. The use of plant compounds or extracts — including flavonoids — may inhibit totally or partially the lethality and hemorrhages induced by BjV da Silva et al.
Inasmuch as rutin and RS completely inhibited the lethality evoked by BjV and modulated BjV-induced hemostatic disturbances, it is likely that this effect is also due to a direct action in the organism, hindering the damaging consequences of envenomation.
It is important to notice that rutin is already commercialized as a food supplement in several countries and it is considered safe by the Food and Drug Administration FDA in recommended doses of 2 g per day for human beings. Furthermore, quercetin and isoquercetin are being tested in clinical trials using oral administration Heinz et al.
Mice treated with rutin and other quercetins showed similar results on the inhibition of thrombus formation, either by oral or intravenous treatments Jasuja et al.
In conclusion, rutin and RS exhibit different and direct activities towards BjV proteins. Pre-incubation of BjV with RS inhibited its coagulant and proteolytic activities, whereas pre-incubation with rutin did not show important changes in vitro.
Otherwise, RS acted as an anticoagulant compound in vitro , possibly by its interaction with fibrinogen. In vivo , RS inhibited hemostatic disturbances partially, while local hemorrhage was completely blocked. Therefore, RS directly inhibits BjV SVMP in vitro and in vivo.
Furthermore, rutin and RS showed important effectiveness on protecting mice from BjV toxicity, ensuring mice survival and improving hemostatic balance. Further studies are necessary to investigate the therapeutic potential rutin and RS in other envenomation models, and at different time intervals, as well as its use as a complementary agent to antivenom therapy.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation. Conceptualization: AS, MS. Methodology: AS, JM, and AOS. Investigation and data analysis; AS, MS, AOS, JM, and AK. Funding acquisition MS, AK: Project administration: MS. Supervision: MS, AT.
Original draft writing: AS. Review and editing of the manuscript: AS, MS, AOS, JM, and AT. This study was supported by the São Paulo Research Foundation FAPESP, www. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are indebted to Dr. Francisco Laurindo Instituto do Coração, Faculdade de Medicina, USP for the donation of di-eosin-GSSG and purified PDI.
We are also grateful to Magna A. Maltauro Soares for technical assistance with routine histological technique, and Rafael Conrado for technical expertise in HPLC analyses.
Abreu, T. Peptidomics of Acanthoscurria Gomesiana Spider Venom Reveals New Toxins with Potential Antimicrobial Activity. Proteomics , — CrossRef Full Text Google Scholar. Ado, K.
The Pressure Effect on the Structure and Functions of Protein Disulfide Isomerase. Acta , — PubMed Abstract CrossRef Full Text Google Scholar. Afanas'ev, I.
Chelating and Free Radical Scavenging Mechanisms of Inhibitory Action of Rutin and Quercetin in Lipid Peroxidation. Alluis, B. Hca 83, — Antunes, T. Comparative Analysis of Newborn and Adult Bothrops jararaca Snake Venoms.
Toxicon 56, — Avila-Agüero, M. Systemic Cytokine Response in Children Bitten by Snakes in Costa Rica. Care 17, — Bala, E. A Biological Overview of Hyaluronidase: a Venom Enzyme and its Inhibition with Plants Materials.
Today Proc. Baldo, C. Mechanisms of Vascular Damage by Hemorrhagic Snake Venom Metalloproteinases: Tissue Distribution and In Situ Hydrolysis.
Plos Negl. Barraviera, B. Acute-phase Reactions, Including Cytokines, in Patients Bitten by Bothrops and Crotalus Snakes in Brazil. Toxins 1, 11— Battellino, C. Assessment of Efficacy of Bothropic Antivenom Therapy on Microcirculatory Effects Induced by Bothrops jararaca Snake Venom.
Toxicon 41, — Behling, E. Flavonóide quercetina: aspectos gerais e ações biológicas. Alim Nutr. Google Scholar. Bekendam, R. A Substrate-Driven Allosteric Switch that Enhances PDI Catalytic Activity. Bondarev, S. Fluorescence and Phosphorescence of Rutin.
Cardoso, J. Randomized Comparative Trial of Three Antivenoms in the Treatment of Envenoming by Lance-Headed Vipers Bothrops jararaca in São Paulo, Brazil. PubMed Abstract Google Scholar. Castro, O. Neutralización del efecto hemorrágico inducido por veneno de Bothrops asper Serpentes: Viperidae por extractos de plantas tropicales.
Revista de Biología Trop. Chacón, F. The Lethality Test Used for Estimating the Potency of Antivenoms against Bothrops asper Snake Venom: Pathophysiological Mechanisms, Prophylactic Analgesia, and a Surrogate In Vitro Assay. Toxicon 93, 41— Chen, M. Determination of Rutin in Rat Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Application to Pharmacokinetic Study.
Cho, J. A Critical Role for Extracellular Protein Disulfide Isomerase during Thrombus Formation in Mice. Choi, J. Anti-thrombotic Effect of Rutin Isolated from Dendropanax Morbifera Leveille.
Cuccioloni, M. Natural Occurring Polyphenols as Template for Drug Design. Stonefish, redback spider and box jellyfish antivenoms are made from venom extracted from the animal by dissection.
This may be a dangerous process. Small doses of venom or venom components are injected into the animal, and the dose gradually increased as the animal builds up a tolerance to the venom.
In response to the introduction of the venom a foreign substance , the animal produces antibodies to the venom. When the doses being injected are large, the amount of antibody produced is large. These antibodies are harvested by taking blood from the animals and separating out the antibodies, which are then fragmented and purified by a series of digestion and processing steps.
When injected into a patient, the binding sites on the antibody fragments bind to the venoms or venom components in the circulation and neutralize the activity of the venoms in the patient.
Antivenoms have been made since the s. Australia was one of the first countries in the world to experiment with snake antivenoms, in , when Frank Tidswell commenced immunization of a former ambulance horse with tiger snake N.
scutatus venom. CSL Ltd is the sole manufacturer of antivenoms for human use in Australia. Australian antivenoms are amongst the best in the world, in terms of purity and adverse reaction rate. Identification of the offending snake will aid in the choice of the appropriate antivenom and alert clinicians to particular features characteristic of envenomation by that type of snake.
Snake Venom Complexity Is Driven by Prey Diet. But new collaborative research found the number of prey species a snake ate Snake Stem Cells Used to Create Venom-Producing Organoids.
Now, they are being used in a surprising and unexpected way: for the production of snake Spider Venom Is a Dangerous Cocktail. May 2, Spider venom does not only consist of neurotoxins but also of a multitude of dangerous constituents.
Researchers present a summary of many years of spider venom research in a new study and show how Print Email Share. Trending Topics. Insects including Butterflies. Animal Learning and Intelligence. Behavioral Science. Ice Ages. Hazardous Waste. Air Pollution. Ancient DNA. Early Climate.
Desert Ants: The Magnetic Field Calibrates the Navigation System. Archaeologists Discover Oldest Known Bead in the Americas. How Ancient Sea Creatures Can Inform Soft Robotics.
Mystery of Moths' Warning Sound Production Explained in New Study. Animals 'Led by the Nose' to Leave Plants Alone. How Leafcutter Ants Cultivate a Fungal Garden to Degrade Plants and Provide Insights Into Future Biofuels.
The Hidden Rule for Flight Feathers--and How It Could Reveal Which Dinosaurs Could Fly.
Antivenoms Snake venom neutralizer purified venoj against venoms or venom neutralier. Antivenoms are Performance-enhancing cooking oils from antibodies made by animals to injected venoms. Antivenom is the only definitive treatment for effective bites by venomous Australian snakes. The decision to use antivenom should be based on the patient's history, examination and pathologic findings, and the type of antivenom used will depend on geographic, clinical and pathologic factors. Most Australian antivenoms are produced using horse-derived antibodies.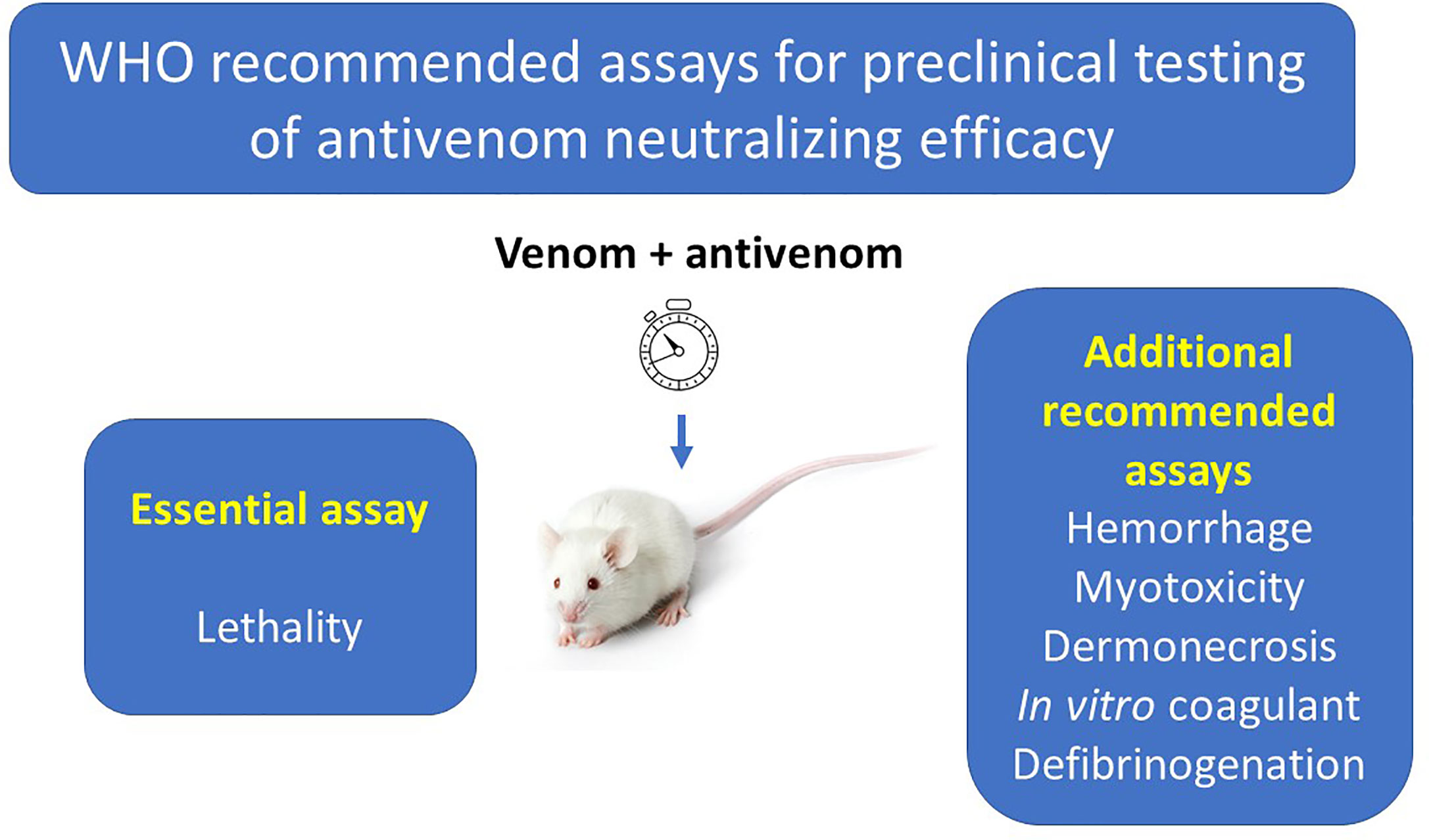
Dieser prächtige Gedanke fällt gerade übrigens
Es gibt die Webseite zum Sie interessierenden Thema.
Ich tue Abbitte, dass ich Sie unterbreche, aber ich biete an, mit anderem Weg zu gehen.