Enhance insulin signaling -
Further, these infusions promote muscle lipid accumulation and effectively induce IR. Consistent with the above studies, elevated plasma fatty acid concentrations can result in increased intracellular diacylglycerol DAG levels, leading to the activation of protein kinase C isoform PKC-θ and PKC-ε isoforms in skeletal muscles and liver respectively.
Since diacylglycerol acyltransferase 1 DGAT1 can increase the conversion of DAG into triacylglycerol TAG , DGAT1 overexpression could decrease DAG levels and improve insulin sensitivity partially attenuating the fat-induced activation of DAG-responsive PKCs.
Taken together, these studies strongly support that DAG as a key intermediate of TAG synthesis from fatty acids has central modulation and potential therapeutic values in IR. Ceramide is another specific lipid metabolite that increases in concentration, along with DAG, in association with IR in obese mice.
Thus, ectopic lipid metabolite concentrations e. Consequently, concerted efforts to decrease lipid components in these organs are the most efficacious therapeutic targets for treating IR and metabolic diseases.
Some human genetic studies indicated that different genomic loci were associated with fasting insulin levels, higher triglyceride and lower HDL cholesterol levels, , which are different hallmarks of IR. The peroxisome proliferator-activated receptor gamma PPARγ variant Pro12Ala was one of the first genetic variants identified that is involved in fatty acid and energy metabolism and that is associated with a low risk of developing T2DM.
Nevertheless, additional studies are needed to assess the functional relationships between the genetic variants and IR, that are also influenced by various lifestyle and environmental factors.
Recent studies have suggested that epigenetic modifications such as DNA methylation DNAm and histone post-translational modifications PTM are implicated in the development of systemic IR. Global and site-specific DNA methylation is generally mediated by DNA methyltransferases DNMTs.
These processes mainly occur in the context of CG dinucleotides CpGs and promoter region, while also involving covalent addition or removal of methyl groups as a means to repress or stimulate transcription, respectively. For example, increased INS promoter methylation levels and INS mRNA suppression were observed under over-nutrition conditions and obese T2DM patients.
Another study demonstrated that increased IGFBP2 DNA methylation levels were are associated with lower mRNA expression levels in Visceral Adipose tissue VAT of abdominal obesity. Moreover, the first global genome-wide epigenetic analysis in VAT from IR and insulin-sensitive IS morbidly obese patients identified a novel IR-related gene, the zinc finger protein ZNF exhibited the highest DNA methylation difference, and its methylation levels is lower in IR patient than in IS patient, consistent with increased transcription levels, such studies provide potential epigenetic biomarkers related to IR in addition to novel treatment targets for the prevention and treatment of metabolic disorders.
For example, peroxisome proliferator-activated receptor-α and -γ PPAR-α and PPAR-γ, respectively are encoded by PPARA and PPARG , respectively, and they are the two primary nuclear peroxisome proliferator-activated receptors involved in lipid metabolism.
Higher PPARA and PPARG methylation levels were observed in association with obesity, consistent with decreased PPAR-α and PPAR-γ protein expression levels, that lead to dyslipidemia and IR. SLC19A1, a gene encoding a membrane folate carrier, was reduced in obese WAT and induced global DNA hypermethylation of chemokine C-C motif chemokine ligand 2 CCL2 that is a key factor in WAT inflammation, resulting in increased CCL2 protein secretion and the development of IR in obese.
In addition, several genes methylation involved in hypoxia stress and endoplasmic reticulum stress were regulated in obesity related metabolic diseases. Recent epigenetic genome-wide analysis identified low HIF3A methylation levels upregulates HIF3A expression in adipose tissue, thereby leading to adipose tissue dysfunction and adiposity.
Ramos-Lopez et al. Specifically, increased insulin concentrations and HOMA-IR index were accompanied by lower ERO1LB and NFE2L2 methylation levels.
The histone modification effect on gene expression mainly includes histones methylation and acetylation. Histone methylation could either activate gene transcription H3K4, H3K36, and H3K79 or silence gene expression H3K9 and H3K27 , which depends on the modification site.
Histone acetylation increases the accessibility and gene expression of various transcription factors by reducing the positive charge and histone affinity for DNA.
Increasing evidence indicates , that IGFR, InsR, IRS1, Akt, GLUT4, and PPAR are more deacetylated in association with IR than in normal physiological conditions. In contrast, IRS2, FoxO, JNK, and AMPK are usually acetylated in association with IR. Castellano-Castillo, D.
Further, global proteomic analyses have revealed 15 histone modifications that are differentially abundant in hepatic IR. MicroRNAs miRNAs are small ncRNAs nucleotides incorporated into Argonaute Ago protein to form miRISCs, which can inhibit the expression of partially or completely complementary target mRMAs.
Several miRNAs are involved in β cell differentiation and mature β cell functioning. For example, islet-specific miR overexpression represses glucose-stimulated insulin secretion GSIS and insulin gene transcription, that is then reversed upon miR inhibition. Thus, these markers may improve disease prediction and prevention in individuals at high risk for T2DM.
Furthermore, pdx1, neurogenin-3 ngn3 , and a transcriptional factor essential for insulin transcription MafA are essential transcription factors for β-cell differentiation. Thus, miRa2 can directly modulate insulin expression through foxA2 and then pdx1.
miR expression is induced by the cellular redox regulator thioredoxin-interacting protein TXNIP that then represses MafA, thereby inhibiting insulin production.
Numerous studies suggest that miRNAs have pivotal roles in glucose and lipid metabolism. miR was first reported to directly regulate GLUT4 expression in adipocytes. In addition, the anti-diabetic drug metformin can up-regulate miRp expression to suppress G6Pase and inhibit hepatic gluconeogenesis.
The balance of low-density lipoprotein LDL and high-density lipoprotein HDL molecules that are synthesized in hepatocytes is critical for lipid homeostasis. Many miRNAs have been identified as critical regulators of HDL and LDL biogenesis. For example, miR, miR, miR, and miRa repress expression of the ATP-binding cassette transporter ABCA1 that mediates hepatic HDL generation.
In addition, miRc targets the gene encoding microsomal triglyceride transfer protein MTP that is required for the lipidation of newly synthesized APOB in the liver for LD lipoprotein production.
miRc overexpression reduces the assembly and secretion of these APOB-containing lipoproteins, resulting in decreased plasma LDL levels. miRa and miR repress LDLR expression and inhibition of these miRNAs results in enhanced LDLR expression and clearance of circulating LDL. Further, miR and miRd target the LDLR chaperonin PCSK9 and IDOL in addition to the rate-limiting enzyme in cholesterol biosynthesis, HMGCR.
Chronic inflammation in insulin-reactive tissues is one of the most important causes of IR and increasing evidence suggests that miRNAs has a pivotal role in the inflammatory process. Obesity inhibited miR expression in adipose tissue macrophages ATMs , and miR was shown to target Delta-like-4 DLL4 , a Notch1 ligand is associated with ATM inflammation.
Conversely, Wang et al. discovered that miRp is significantly upregulated in Natural killer NK cells-derived exosomes from lean mice, which directly targets SKI family transcriptional corepressor 1 SKOR1 , subsequently downregulated the expression levels of pro-inflammatory cytokine factors including IL-1β, IL-6, and TNF-α levels and attenuated IR.
Therefore, it might be that metabolism-regulating miRNAs play a vital role in the dynamics of metabolic homeostasis. Long non-coding RNAs lncRNAs are non-coding transcripts more than nucleotides, and the subcellular localization of lncRNAs determines their function.
LncRNAs located in the nucleus could affect chromosomal biology or interact with transcription factors to regulate gene transcription; lncRNAs located in cytosol could modulate mRNA stability and translational efficiency by acting as sponges for miRNAs or direct pairing with mRNA.
Recent advances have shown that lncRNAs play crucial roles in the pathologys of IR and diabetes. Glucose and lipid metabolism disorders are the primary causes for the pathophysiological development of IR.
The lncRNA SRA promotes insulin-stimulated glucose uptake by co-activating PPARγ, leading to increased phosphorylation of the downstream targets Akt and FOXO1 in adipocytes. These processes are closely related to the genes PGC1a and CPT1b that reverse FFA-induced lipid accumulation and improve IR.
In addition the insulin target tissues, transcriptome profiling and different studies have identified several β-cell specific lncRNAs that contribute to obesity-mediated β-cell dysfunction and apoptosis.
LncRNA MALAT1 downregulation may lead to pancreatic β-cell dysfunction and T2DM development by direct interaction and regulation of polypyrimidine bundle binding protein 1 PTBP1.
Further, lncRNA-p overexpression can decrease the β cell apoptosis ratio and partially reverse the glucotoxicity effects on GSIS function.
Contrary to conventional linear RNA, circRNAs are noncoding RNAs that generated from precursor mRNAs by back-splicing circularization, which is derived from exonic circRNAs, intronic circRNAs, exonic-intronic circRNAs and ntergenic circRNAs. Recent studies have suggested that newly identified circRNAs are novel factors in the initiation and development of IR.
CircHIPK3 is one of the most abundant circRNAs in β-cells and regulates hyperglycemia and IR by sequestering miRp and miRp, thereby increasing mRNA expression of key β-cell genes e. Similar to the miRNAs and lncRNAs, several circRNAs also contribute to the the regulation of glucose and lipid homeostasis.
Deep sequencing analysis of adipose circRNA revealed that circArhgap is highly upregulated during differentiation of human white adipocytes. Thus, circRNAs likely serve as important regulators of adipocyte differentiation and lipid metabolism. Another circRNA deep sequencing analysis of sera from patients with metabolic syndrome MetS identified the presence of a novel circRNA, circRNF, involved in MetS progression.
AMPK is a critical factor in energy homeostasis including glycolysis, lipolysis, and fatty acid oxidation FAO. CircACC1 is a circRNA derived from the human acetyl-CoA carboxylase 1 ACC1 gene and directly binds to the β and γ subunits of AMPK, facilitating its activity, and promoting glycolysis and fatty acid β-oxidation during metabolic stress.
circMAP3K4 is another potentially important circRNA involved in glucose metabolism that is highly expressed in the placentas of patients with gestational diabetes mellitus GDM and the IR model. Nevertheless, the exact roles and regulatory mechanisms of circRNAs in IR require additional clarity.
The microbes living in the human gut are key contributors to host metabolism and immune function through mediating the interaction between the host and environment, or releasing metabolites and cytokines. Different factors influencing these alterations of gut microbiome composition have been explored including diet, exercise, circadian disruption, antibiotics treatments, and genetics.
The gut microbial communities of the groups significantly diverged over time, with participants on animal diets experiencing proliferation of bile-tolerant microorganisms e. For example, microbiome genome-wide association studies mGWAS have identified that variants of different genes for example, VDR , LCT , NOD2 , FUT2 , and APOA5 that are associated with distinct gut microbiome compositions.
Growing evidence in the last two decades has suggested that gut microbial dysbiosis contributes to increased risks of metabolic defects like obesity, IR, and diabetes.
LPS circulation then contributes to the chronic inflammation of liver and adipose tissue that is associated with the development of IR, in addition to other conditions associated with metabolic syndromes.
As we all know, IR is a state in which higher than normal concentrations of insulin are needed for a normal response, leading directly to hyperinsulinaemia and impaired glucose tolerance. Non-alcoholic fatty liver disease NAFLD is one of the most common liver diseases worldwide.
Adipose tissue is a physiologic reservoir of fatty acids, when the storage capacity is exceeded, the accumulation of heterotopic lipids leads to lipotoxicity, thereby promoting low-grade inflammation and IR in the liver.
Lipotoxic injury appears to occur in response to excessive levels of serum free fatty acids FFAs in hepatocytes. At present, the molecular mechanism of insulin in PCOS has been well described.
Such modifications then activate NF-κB that is involved in the expression of proinflammatory mediators such as TNF and IL-6, , and that induces key steroidogenic molecules, like CYP11A1, CYP17A1 and StAR, leading to further aggravation of hyperandrogenemia. Cardiovascular diseases CVDs are the leading causes of death globally.
The World Health Organization estimates that Moreover, over 23 million people are estimated to die from CVDs each year by However, the most common types of CVDs include high blood pressure, coronary artery disease CAD , stroke, cerebrovascular disease and rheumatic heart disease RHD.
Identifying new therapies to reduce IR may contribute to the reduced prevalence of CVDs. Insulin primarily enters the brain via selective, saturable transport across the blood-brain barrier BBB , , Peripherally produced insulin can also be actively transported into the brain via an endocytic-exocytic mechanism.
Current researches have demonstrated that the mechanisms of systemic IR and brain-specific IR have close links with AD pathogenesis. Pioglitazone acts similarly as Rosiglitazone by reducing tau and Aβ deposits in the hippocampus, and improving neuronal plasticity and learning in AD.
Moreover, overlapping pathological features exist for diabetes, IR, and AD. Chronic kidney disease CKD involves a gradual loss of kidney function and inability to filter blood , and is a major risk factor for end-stage kidney failure ESKF and CVDs. Numerous recent epidemiological studies have suggested that IR increases the risks for different cancers including colon, liver, pancreas, breast, endometrium, thyroid and gastric cancer.
Further, a growing body of evidence suggests that increased insulin, in addition to IGF1 and IGF2 levels critically influence tumor initiation and progression in IR patients.
As we all know, IR is related to several metabolic abnormalities including obesity, glucose tolerance, dyslipidemia, type 2 diabetes and other metabolic syndrome. Actually, IR precedes the occurrence of T2DM, so how to increase the accurate assessment of insulin sensitivity is very important to predict the risk and evaluate the management of impaired insulin sensitivity and metabolic syndrome in research and clinical practice.
HOMA2 updated HOMA model which took account of variations in hepatic and peripheral glucose resistance , homeostatic Model Assessment for IR HOMA-IR , the oral glucose insulin sensitivity index OGSI , fasting Insulin FINS , and fasting plasma glucose FPG based on fasting glucose and insulin levels , , , , are widely utilized IR measurements in clinical research.
Other indices based on fasting insulin include the glucose to insulin ratio GIR , the quantitative insulin sensitivity check index QUICKI , , , triglycerides McAuley Index alone or in accordance with HDL cholesterol HDL-C , whole-body insulin sensitivity index WBISI , Matsuda Index to evaluate whole body physiological insulin sensitivity by the above methods.
Indeed, the early symptoms of IR in different individuals are not obvious, and the related symptoms are very complex, combining with screening indicators may provide more precise diagnosis for IR in the general population. No medications exist currently that are specifically approved to treat IR, but IR management 91 , , is possible through lifestyle changes like dietary, increased exercise, and disease prevention in addition to alternative medications Fig.
Among these treatments, lifestyle changes should be the main focus for IR treatment, with nutritional intervention to decrease calories, avoidance of carbohydrates, and focusing on aliments with low glycemic index including vegetables, fruits, whole-grain products, nuts, lean meats or beans to provide higher fiber, vitamins, healthy fats and protein are particularly helpful for people trying to improve insulin sensitivity.
Table 1. Metformin is a first-line medication and the most widely-prescribed insulin-sensitizing agent in T2DM and PCOS patients. For example, 1 Glucagon-like peptide 1 GLP1 is an intestinal hormone that can enhance insulin secretion in a glucose-dependent manner by activating the GLP-1 receptor GLP-1R that is highly expressed on islet β cells.
are now world-wide therapy of T2DM since and could improve insulin sensitivity. In clinical research, scientists and physicians have explored different strategies to prevent and treat diabetes mellitus and IR. gov to reduce IR and summarized them mainly include: 1 Diet intervention, such as Low-fat vegetarian Food, high-protein food, calorie restriction, vitamin D supplementation to reduce the IR in human obesity.
We present some clinical trials of IR intervention in Table 2. Over the past years, our knowledge of the pathogenesis of IR and T2DM has improved, the development of new treatments of IR and metabolic syndrome have gained certain success, while the complexity of IR and the presence of multiple feedback loops make a challenge to the specific intervention.
In recent years, accumulating preclinical studies on the intervention of IR have been reported, which have important reference significance for the development of new drugs. We present the related studies on IR reported in recent years in Table 3 , including animal models, treatment methods and results.
Pre-clinical IR intervention mainly includes drug intervention, probiotic therapy and exercise supplement. Drug therapy to improve IR is the main research direction at present. Researchers found that Valdecoxib VAL can inhibit inflammation and endoplasmic reticulum ER stress through AMPK-regulated HSPB1 pathway, thus improving skeletal muscle IR under hyperlipidemia.
The researchers found that the mixed nasal administration of GLP-1 receptor agonist and L-form of peneracin can effectively alleviate the cognitive dysfunction of SAMP8 mice.
Natividad et al. Regular exercise is an alternative intervention measure to maintain the blood sugar level in the normal range and reduce the risk factors. Hsu and colleagues found that exercise combined with probiotics intervention can have a positive effect on blood sugar and increase insulin sensitivity in mice.
The above results show that drug intervention, probiotic supplementation and intensive exercise can improve IR but more clinical data are still needed. Overall, the increased incidence of IR and the key roles of IR plays in many diseases, urgently require a better understanding of IR pathogenesis in addition to how IR interacts with genetics and different environments.
A deeper understanding of IR can be achieved with a more systematic approach involving large-scale omics to study the molecular landscape is of major importance in addition to exploring new intervention strategies to prevent abnormal IR syndrome.
Banting, F. The internal secretion of the pancreas. Indian J. CAS PubMed Google Scholar. SANGER, F. The amino-acid sequence in the glycyl chain of insulin.
The identification of lower peptides from partial hydrolysates. Biochem J. Article CAS PubMed PubMed Central Google Scholar. The investigation of peptides from enzymic hydrolysates.
Kung, Y. Total synthesis of crystalline bovine insulin. Goeddel, D. et al. Expression in Escherichia coli of chemically synthesized genes for human insulin.
USA 76 , — Vecchio, I. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol. Article Google Scholar. Cheatham, B. Insulin action and the insulin signaling network. Root, H. Insulin resistance and bronze diabetes.
Laakso, M. Insulin resistance and hyperglycaemia in cardiovascular disease development. Article CAS PubMed Google Scholar.
Bugianesi, E. Insulin resistance in nonalcoholic fatty liver disease. Saklayen, M. The global epidemic of the metabolic syndrome. Diamanti-Kandarakis, E. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications.
Stenvers, D. Circadian clocks and insulin resistance. Article PubMed CAS Google Scholar. Freeman AM, Pennings N. Insulin Resistance. In: StatPearls Internet.
Treasure Island FL : StatPearls Publishing. PMID: American Diabetes Association. Prevention or delay of type 2 diabetes: standards of medical care in diabetes Diabetes Care 44 , S34—S39 Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes Diabetes Care 44 , S—S Weiss, M.
Insulin biosynthesis, secretion, structure, and structure-activity relationships. In: Feingold KR, Anawalt B, Boyce A, et al.
South Dartmouth MA : MDText. com, Inc. Sanger, F. Chemistry of insulin. Science , — Katsoyannis, P. Synthesis of insulin. Lee, J. The insulin receptor: structure, function, and signaling. Pessin, J. Signaling pathways in insulin action: molecular targets of insulin resistance.
Invest , — Haeusler, R. Biochemical and cellular properties of insulin receptor signalling. Cell Biol. White, M. Mechanisms of insulin action. In Atlas of diabetes pp. Springer, Boston, MA Newgard, C. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase Diabetes 49 , — Beurel, E.
Glycogen synthase kinase-3 GSK3 : regulation, actions, and diseases. Article CAS Google Scholar. Dong, X. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation.
Cell Metab. Puigserver, P. Insulin-regulated hepatic gluconeogenesis through FOXO1—PGC-1α interaction. Nature , — Vander Haar, E. Garami, A. Cell 11 , — Laplante, M. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis.
USA , — Han, Y. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Calejman, C. mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis. Xia, W. Loss of ABHD15 impairs the anti-lipolytic action of insulin by altering PDE3B stability and contributes to insulin resistance.
Cell Rep. James, D. The aetiology and molecular landscape of insulin resistance. Tam, C. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes care 35 , — Samuel, V. Mechanisms for insulin resistance: common threads and missing links.
Cell , — Ye, J. Mechanisms of insulin resistance in obesity. Front Med. Article PubMed PubMed Central Google Scholar. Yaribeygi, H. Insulin resistance: Review of the underlying molecular mechanisms. Cell Physiol. De Meyts, P. The insulin receptor: a prototype for dimeric, allosteric membrane receptors?
Trends Biochem Sci. Caro, J. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. Invest 79 , — Fröjdö, S. Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans.
Biochim Biophys. Acta , 83—92 Fisher, S. Michael, M. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Cell 6 , 87—97 Davis, R. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser Article PubMed Google Scholar.
Carvalho-Filho, M. Diabetes 54 , — Taniguchi, C. Critical nodes in signalling pathways: insights into insulin action. Brachmann, S. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice.
Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. CAS PubMed PubMed Central Google Scholar. Czech, M. Signaling mechanisms that regulate glucose transport.
Luo, J. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cong, L. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells.
Xia, J. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Le Marchand-Brustel, Y. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice.
Brozinick, J. Defective signaling through Akt-2 and-3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes 52 , — Kruszynska, Y. Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation.
Choi, K. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J. Intern Med. Kahn, B.
Glucose transport: pivotal step in insulin action. Diabetes 45 , — Dimitriadis, G. Insulin effects in muscle and adipose tissue.
Diabetes Res Clin. Shepherd, P. Glucose transporters and insulin action-implications for insulin resistance and diabetes mellitus. Li, J. Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice.
FASEB J. Klip, A. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem Biophys. Res Commun. Etgen, G. Jr et al.
Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to the cell surface. Ryder, J. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin-and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients.
Garvey, W. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes: heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters.
Diabetes 42 , — Chadt, A. Deletion of both Rab-GTPase—activating proteins TBC14KO and TBC1D4 in mice eliminates insulin-and AICAR-stimulated glucose transport. Diabetes 64 , — Chen, S. Tramunt, B. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63 , — Greenhill, C.
Sex differences in insulin resistance. Qiu, J. Estradiol protects proopiomelanocortin neurons against insulin resistance. Endocrinology , — Zidon, T.
Effects of ERβ and ERα on OVX-induced changes in adiposity and insulin resistance. Ikeda, K. Functions of estrogen and estrogen receptor signaling on skeletal muscle. Steroid Biochem Mol. Gerdts, E. Sex differences in cardiometabolic disorders.
Chia, C. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Shi, H. Sex differences in obesity-related glucose intolerance and insulin resistance.
Glucose Tolerance 4 , 37—66 Google Scholar. Geer, E. Gender differences in insulin resistance, body composition, and energy balance. Christen, T.
Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides , 25—31 Palmisano, B. Sex differences in lipid and lipoprotein metabolism. Kodama, K. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis.
Diabetes Care 36 , — Raygor, V. Diab Vasc. Sumner, A. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis , — Tan, V. Ethnic differences in insulin sensitivity and beta-cell function among Asian men.
Diabetes 5 , e—e Ministry of Health Singapore MOHS. Potts, J. Sex and ethnic group differences in fat distribution in young United Kingdom South Asians and Europids. Ehtisham, S. Ethnic differences in insulin resistance and body composition in United Kingdom adolescents.
Lear, S. Ethnic variation in fat and lean body mass and the association with insulin resistance. Mason, C. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Med 41 , — Mikusova, V.
Insulin resistance and need for a lifestyle change to eliminate it. Listy , — orpeleijn, E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Shigeta, H. Lifestyle, obesity, and insulin resistance.
Diabetes Care 24 , Oosterman, J. The circadian clock, shift work, and tissue-specific insulin resistance. Endocrinology , bqaa McAuley, K. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial.
Diabetes care 25 , — Bergman, B. Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes 61 , — Kan, C. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes care 36 , — Sung, C.
Role of vitamin D in insulin resistance. Ardabili, H. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Pasieka, A. Impact of glucocorticoid excess on glucose tolerance: clinical and preclinical evidence.
Metabolites 6 , 24 Article PubMed Central CAS Google Scholar. Rizza, R. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action.
Effects of growth hormone on insulin action in man: mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization.
Diabetes 31 , — Barbour, L. A Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30 , S—S Parichatikanond, W.
Prolonged stimulation of β2-adrenergic receptor with β2-agonists impairs insulin actions in H9c2 cells. Walli, R. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIVinfected patients. AIDS 12 , F—F Murata, H. The mechanism of insulin resistance caused by HIV protease inhibitor therapy.
Teff, K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes 62 , — Bittencourt, M. Insulin therapy in insulin resistance: could it be part of a lethal pathway?
Elbein, S. Heritability of pancreatic beta-cell function among nondiabetic members of Caucasian familial type 2 diabetic kindreds. Shulman, G. Cellular mechanisms of insulin resistance. Knauf, C. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage.
Petersen, M. Regulation of hepatic glucose metabolism in health and disease. Matsumoto, M. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. Shimomura, I. Cell 6 , 77—86 Petersen, K. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans.
Henriksen, E. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Drug Targets 7 , — Karim, S. Hepatic expression and cellular distribution of the glucose transporter family. World J. Rencurel, F. Requirement of glucose metabolism for regulation of glucose transporter type 2 GLUT2 gene expression in liver.
Thorens, B. Diabetologia 58 , — Eberlé, D. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86 , — Horton, J. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Ferré, P. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c.
Diabetes Obes. Tobe, K. Dentin, R. Carbohydrate responsive element binding protein ChREBP and sterol regulatory element binding protein-1c SREBP-1c : two key regulators of glucose metabolism and lipid synthesis in liver.
Biochimie 87 , 81—86 Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression.
Herman, M. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Iizuka, K. Deficiency of carbohydrate response element-binding protein ChREBP reduces lipogenesis as well as glycolysis. Natl Acad. Jaworski, K. Regulation of triglyceride metabolism.
Hormonal regulation of lipolysis in adipose tissue. Liver Physiol. Vaughan, M. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. Zmuda-Trzebiatowska, E. Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes.
Cell Signal 18 , — Choi, Y. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B—null mice. Martinez-Botas, J. Genet 26 , — Tansey, J. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity.
USA 98 , — Mechanisms of Insulin Action and Insulin Resistance. Kimball, S. Regulation of protein synthesis by insulin. Pösö, A. Multifunctional control of amino acids of deprivation-induced proteolysis in liver. Role of leucine. Marshall, S. New insights into the metabolic regulation of insulin action and insulin resistance: role of glucose and amino acids.
Rudrappa, S. Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance-a qualitative review.
Front Physiol. Medeiros, C. Antuna-Puente, B. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. Rabe, K. Adipokines and insulin resistance. Adipokines mediate inflammation and insulin resistance.
Lausanne 4 , 71 Li, S. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA , — Hotta, K. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys.
Diabetes 50 , — Takahashi, M. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. Yamauchi, T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions.
Li, L. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects.
Diabetes , — Soriguer, F. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Yue, P. Apelin is necessary for the maintenance of insulin sensitivity. American journal of physiology.
Apelin decreases lipolysis via G q , G i , and AMPK-dependent mechanisms. Endocrinology , 59—68 Segal, K. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men.
Amitani, M. The role of leptin in the control of insulin-glucose axis. The phosphorylation of Foxo1 at S by Akt promotes Foxo1 cytoplasmic retention and ubiquitination, which serve as a central mechanism controlling Foxo1 stability and activity Guo However, Foxo1 can also be phosphorylated at different serine or threonine residues by other protein kinases, enhancing transcriptional activity.
For example, mammalian sterile like kinase 1 MST1 promotes Foxo1 phosphorylation at S , which promotes neuronal cell apoptosis Yuan et al. In addition to the phosphorylation-based pathway, the activity of Foxo1 can also be regulated by other post-translational modifications, including methylation, glycosylation, and acetylation Fig.
Human Foxo1 phosphorylation, ubiquitination, methylation, acetylation, and glycosylation at amino acid residues via different pathways and enzymes. However, whether PRMT1 expression and Foxo1 methylation are altered in diabetics is unclear.
The glycosylation of Foxo1 at threonine T via O -GlcNac modification in response to glucose increased Foxo1 transcriptional activity for the expression of gluconeogenic genes Pepck and G6pase and anti-oxidative stress genes Mnsod Sod2 and catalase Housley et al.
The flux of glucose through the hexosamine biosynthetic pathway provides a substrate for the glucosaminephosphate forming UDP-GlcNAc UDP- N -acetylglucosamine. O -GlcNAc modification of proteins results in an enzymatic addition of the N -acetyl glucosamine GlcNAc moiety of UDP-GlcNAc on the hydroxyl oxygen of serines and threonines Kuo et al.
Foxo1-T is GlcNAcylated in the liver and it is a modification that is increased in diabetic animals Housley et al.
In contrast, recent studies indicate that the acetylation of Foxo1 suppresses Foxo1 activity, while deacetylation by SIRT1 increases it Matsuzaki et al.
Moreover, Foxo1 is deacetylated and activated by class IIa histone deacetylases HDACs , promoting hepatic glucose production Mihaylova et al.
Nuclear HDAC4, HDAC5, and HDAC7 are phosphorylated and excluded from the nucleus by AMP-dependent protein kinase AMPK , but fasting hormone glucagon rapidly dephosphorylates and translocates the HDACs to the nucleus, where they associate with the promoters of gluconeogenic enzymes, such as Pepck and G6pase.
In turn, HDAC4 and HDAC5 recruit HDAC3, which results in acute transcriptional induction of these genes via the deacetylation and activation of Foxo transcription factors.
The loss of class IIa HDACs in murine liver results in the inhibition of Foxo target genes and lowers blood glucose levels Mihaylova et al. Moreover, with food intake, cells accumulate acetyl-CoA from glucose oxidation, providing substrate for the acetylation of Foxo1 and suppression of Foxo1 activity, in addition to insulin-induced inhibitory phosphorylation.
Thus, Foxo1 merges the nutritional and hormonal signaling into a well-controlled metabolic regulation Fig. It is of note that Foxo1 stimulates the expression of manganese superoxide dismutase MnSOD and catalase and enhances antioxidant responses.
In rodents, the activation of Foxo1 following Irs2 deficiency in the brain enhanced longevity, but promoted obesity and diabetes Taguchi et al.
Also, the activation of Foxo1 enhanced myocardial survival upon the induction of oxidative stress Sengupta et al. Mice lacking systemic Foxo1 display embryonic lethality, since Foxo1 is required for endothelial cell lineage during cardiovascular development Hosaka et al.
Together, these data indicate that the activation of Foxo1 is required for the maintenance of the life cycle under stressful conditions, such as prolonged fasting, in the liver for hepatic glucose production and activation of anti-oxidative mechanisms promoting survival in C.
However, Foxo1 is activated through multiple layers of regulatory mechanisms, contributing to the development of type 2 diabetes mellitus and organ failure, following insulin resistance.
Human appetite is tightly controlled by the action of insulin in the CNS. The hypothalamus at the base of the forebrain comprises numerous small nuclei, each with distinct connections and neurochemistry, which regulate food intake, hormone release, sleep and wake cycles, and other biological functions.
A low dose of insulin delivery by i. infusion decreased both food intake and hepatic glucose production, effects which were blocked by PI3K inhibitors Woods et al.
Combined with evidence that mice with neuron-specific Ir deletion are overweight and insulin resistant Bruning et al. The functional significance of brain insulin signaling is further evidenced by the deletion of Irs2 in the hypothalamus resulting in hyperglycemia and obesity in mice Lin et al.
However, both leptin and insulin promoted IRS2 tyrosine phosphorylation and PI3K activation in the brain Warne et al. Hypothalamic neurons expressing Agouti-regulated peptide Agrp stimulate food intake orexigenic: appetite stimulant during the fasting state.
Foxo1 stimulates orexigenic Agrp expression, an effect reversed by leptin delivery, in which the activation of Stat3 abrogates Foxo1 occupancy on the Agrp promoter region Kitamura et al. The deletion of Foxo1 in AGRP neurons of mice resulted in reduced food intake, leanness, and decreased hepatic glucose production, involving the suppression of a G-protein-coupled receptor Gpr17 , a Foxo1 target gene in AGRP neurons Ren et al.
By antagonizing the effect of Agrp, hypothalamic neurons expressing pro-opiomelanocortin Pomc inhibit food intake during the feeding state anorexic: lack of appetite.
The deletion of Foxo1 in POMC neurons resulted in reduced food intake and body weight, by increasing the expression of obesity susceptibility gene, carboxypeptidase E Cpe , and subsequent production of β-endorphin, which mediates anorexigenic effects in mice Plum et al.
A key feature of metabolic syndrome is hyperlipidemia, which probably results from insulin resistance in adipose tissue. Insulin promotes fat cell differentiation, enhances adipocyte glucose uptake, and inhibits adipocyte lipolysis.
Mice lacking adipocyte Torc2 exhibited hyperglycemia, hyperinsulinemia, failure to suppress lipolysis in response to insulin, elevated circulating fatty acid and glycerol levels, and insulin resistance in the skeletal muscle and liver Kumar et al.
These data indicate that when insulin action fails in the adipose tissue, adipocyte development is retarded and lipids are unable to convert from carbohydrates for storage. Thus, both glucose and lipids will redistribute into the circulation and organs, resulting in hyperlipidemia and fatty organs.
These studies significantly underscore the contribution of insulin resistance in adipose tissue, via the inactivation of Akt signaling, to the control of systemic nutrient homeostasis. Adipose tissue is also an endocrine organ secreting cytokines and hormones, including TNFα TNF , IL6, leptin, adiponectin, and many other factors, influencing food intake, systemic insulin sensitivity, and nutrient homeostasis.
However, obesity from fat expansion disrupts a proper balance of cytokine and hormone generation, promoting insulin resistance. For example, TNFα, IL6, and leptin are pro-inflammatory factors and their levels are markedly increased in obesity, where the levels of adiponectin, which has anti-inflammatory effects on the enhancement of insulin sensitivity, are markedly reduced Hotamisligil et al.
The overexpression of IKKb for the activation of NFκB a key player in the control of pro-inflammatory responses in the liver of mice is sufficient for inducing insulin resistance and type 2 diabetes Cai et al. TNFα reduces IRS1 protein levels by the activation of JNK or S6K, resulting in insulin resistance Gao et al.
Thus, the suppression of inflammation increases insulin sensitivity and reduces metabolic dysfunction in type 2 diabetes mellitus Hotamisligil et al.
However, the outcome of anti-inflammatory therapy in treating insulin resistance deserves a cautionary note for several reasons, which are as follows: i inflammation is involved in the deployment and mobilization of immune cell leukocytes to defend against infections or toxins.
The overexpression of IL6, in the liver, increased energy expenditure and insulin sensitivity in mice Sadagurski et al. ii During physical exercise, inflammatory factors, such as TNFα and IL6, are secreted resulting in the inhibition of anabolic metabolism insulin action and promoting catabolic metabolism fat lipolysis to meet the fuel requirements of the muscle.
iii NFκB is essential for hepatocyte proliferation and survival, and mice lacking the p65 subunit of NFκB die of liver failure Geisler et al. iv Inflammation not only triggers pro-inflammatory responses, but also activates anti-inflammatory processes.
Together, these data indicate that a balance between inflammation and anti-inflammation is required for proper insulin actions and nutrient homeostasis. Thus, correcting the imbalance of hormones, nutrients, and inflammation may provide opportunities and challenges for the prevention and treatment of metabolic syndrome and type 2 diabetes.
In general, excess energy storage in tissues, particularly lipids, is now believed to be a primary factor contributing to metabolic syndrome Reaven a. Free fatty acids derived from nutritional intake or conversion from carbohydrates not only act as an important energy source, but also act as signaling molecules in the modulation of intracellular protein kinases PKC, JNK, etc.
for the inactivation of insulin signaling Oh et al. Excess lipid accumulation in several organs, including adipose tissue, liver, muscle, heart, and blood vessels, results in insulin resistance and triggers metabolic inflammation, a low-grade and chronic inflammatory response Samuel et al.
An acute lipid or fatty acid infusion or chronic HFD directly induces insulin resistance in mice via the activation of PKCθ Griffin et al. Saturated fatty acids also interact with a liver-secreted glycoprotein fetuin A that binds and activates Toll-like receptor 4, resulting in NFκB activation Pal et al.
In contrast, unsaturated fatty acids interact with the G-protein-coupled receptor GRP, inhibiting inflammation and obesity and increasing insulin sensitivity Ichimura et al.
In the liver, lipid accumulation hepatic steatosis is a risk factor for non-alcoholic steatohepatitis, fibrosis, cirrhosis, and liver cancer Kumashiro et al.
Hyperglycemia is caused by insulin resistance not only in the brain and adipose tissue, but also in the liver, which is a central organ controlling blood glucose and lipid homeostasis. Insulin promotes the synthesis of the macromolecules glycogen, lipids and protein in the liver and suppresses hepatic glucose production by inhibiting gluconeogenesis.
The deletion of either Irs1 or Irs2 in the liver maintained glucose homeostasis, but the deletion of both Irs1 and Irs2 L-DKO mice blocked the induction of Akt and Foxo1 phosphorylation by insulin or feeding and resulted in unrestrained gluconeogenesis for hepatic glucose production, resulting in hyperglycemia, with a reduction in hepatic lipogenesis and blood lipid levels Kubota et al.
Moreover, a HFD severely impaired IRS2 expression and tyrosine phosphorylation in the hepatocytes of liver-specific Irs1 null mice and the mice developed severe diabetes Guo et al. Overnutrition or a HFD can modify intracellular signaling, affecting IRS2 expression and functionality, altering metabolic gene expression, and impairing glucose homeostasis.
Hepatic insulin resistance also results in insulin resistance in other tissues, which is demonstrated in L-DKO mice. The L-DKO mice exhibited not only inhibition of the hepatic Akt signaling cascade, but also blunted brain i. insulin action on the reduction of hepatic glucose production in i. clamp experiments Guo et al.
Moreover, L-DKO mice exhibited features of heart failure, probably secondary to hyperinsulinemia, resulting in cardiac IRS1 and IRS2 suppression Qi et al.
Similarly, mice lacking hepatic Ir displayed pro-atherogenic lipoprotein profiles with reduced HDL cholesterol and VLDL particles, and within 12 weeks of being placed on an atherogenic diet, they developed severe hypercholesterolemia Biddinger et al.
These data indicate that hepatic insulin resistance is sufficient to produce dyslipidemia and increased risk of atherosclerosis and cardiac dysfunction.
The role of Foxo1 activation in the control of the development of diabetes is supported by findings in L-TKO mice, which lack Irs1 , Irs2 , and Foxo1 genes in the liver. L-TKO mice demonstrated a significant reversal of elevated blood glucose levels, glucose intolerance, and the fasting—feeding effect on hepatic gene expression, which were observed in L-DKO mice Dong et al.
Similarly, mice lacking both Akt1 and Akt2 in the liver Akt-DLKO or lacking Pdk1 or Mtorc2 which blocks Akt activation developed a similar diabetic phenotype to that seen in L-DKO mice Mora et al. Moreover, mice lacking Akt1 , Akt2 , and Foxo1 TLKO rescued diabetes in the Akt-DLKO mice Lu et al.
It is of interest that, L-TKO and TLKO mice had normal glucose tolerance and responses to the fasting—feeding challenge and suppressed Pepck and G6Pase gene expression to a degree similar to that of control mice Chai et al.
It is likely that hepatic Foxo1 deletion may sensitize brain insulin signaling to reduce hepatic glucose production, even though Akt activity is not controlled. The loss of Irs1 and Irs2 in the liver and brain resulted in hyperglycemia, while loss in other tissues, such as the heart and pancreas, resulted in organ failure.
Thus, it is likely that diabetes may serve as a link to the development of heart failure via the loss of IRS proteins. The heart is an insulin-responsive and energy-consuming organ that requires a constant fuel supply to maintain intracellular ATP levels for myocardial contraction.
The deletion of both cardiac Irs1 and Irs2 H-DKO mice: heart-specific double Irs1 and Irs2 gene knockout diminished cardiac Akt and Foxo1 phosphorylation and resulted in heart failure and death of male animals at 7—8 weeks of age Qi et al.
The deletion of both Irs1 and Irs2 in the skeletal and cardiac muscle caused heart failure and diminished Akt and Foxo1 phosphorylation in the skeletal muscle, but the mice had normal blood glucose levels and insulin sensitivity Long et al. In contrast, cardiac muscle requires either IRS1 or IRS2 for the maintenance of endogenous Akt activity and Foxo1 inactivation to promote cardiac function and survival.
The overexpression of cardiac Foxo1, which caused heart failure in mice Evans-Anderson et al. The loss of Irs1 and Irs2 following chronic insulin stimulation and p38 MAK activation contributes to insulin resistance in the heart Qi et al. Based on our recent studies, we proposed that the regulation of IRS1 and IRS2 has a major role in the control of cardiac homeostasis, metabolism, and function.
This concept was based on the following observations: i metabolic adaptation during physiological conditions phase I ; ii metabolic remodeling following the development of insulin resistance and mild cardiac dysfunction phase II ; and iii maladaptive metabolic and cardiac remodeling, leading to cardiac failure and sudden death phase III.
During phase I in the postprandial setting, insulin stimulates glucose transport and oxidation, resulting in effective cardiac utilization of glucose as a substrate for the supply of ATP.
In phase II when insulin resistance occurs, the heart undergoes adaptive responses to limit glucose utilization insulin-dependent and responds to lipid oxidation less insulin-dependent.
The heart is capable of generating ATP for myocardial contraction and changes in gene expression patterns, with unaltered cardiac morphology. During this period, the metabolic adaptation or remodeling compensates for cardiac energy demand, even without overt indications of heart failure.
During phase III in H-DKO mice, when maladaptive metabolic remodeling occurs, there is a lack of compensation for cardiac energy demand, secondary to the loss of Irs1 and Irs2 , with Akt inactivation, utilization of both glucose and fatty acids being restrained, resulting in hyperlipidemia and cardiac ATP deficiency and sudden death Qi et al.
In this phase, the failing heart may exhibit a loss of mitochondrial biogenesis, a process required for fatty acid and glucose utilization via mitochondrial oxidative phosphorylation. In addition, unknown myocardial factors, which are derived from the loss of Irs1 and Irs2 and released to cardiofibroblasts, may also contribute to the onset of interstitial fibrosis.
Pancreatic β-cell failure is essential for the development of hyperglycemia in type 1 diabetes, but β-cell failure is also observed in patients with type 2 diabetes Rhodes , Rhodes et al.
The β-cells secret insulin, reducing blood glucose levels, and the α-cells secret glucagon, increasing blood glucose levels to meet bodily metabolic requirements. Recent studies have shown that insulin enhances glucose-stimulated insulin secretion in healthy humans Bouche et al.
However, whether insulin has a direct autocrine action on β-cells in promoting insulin secretion is unclear Rhodes et al. The deletion of whole-body Irs2 in mice resulted in diabetes owing to pancreatic β-cell failure Withers et al.
On the other hand, the deletion of Irs2 in β-cells triggered β-cell repopulation or regeneration, leading to a restoration of insulin secretion and resolution of diabetes in aged mice Lin et al. Conversely, the inactivation of Foxo1 in β-cells resulted in reduced β-cell mass, hyperglycemia, and hyperglucagonemia, owing to the dedifferentiation of β-cells into progenitor-like cells or pancreatic α-cells Talchai et al.
On the other hand, antagonizing glucagon receptor action in type 1 diabetes induced by streptozotocin and type 2 diabetes mellitus in mice markedly reduced blood glucose levels and completely prevented diabetes Liang et al.
Thus, an abnormality at the level of the pancreas is critical for the development of diabetes, and the correction of the imbalance of hormones between insulin β-cells and glucagon α-cells may provide a potential strategy to prevent diabetes.
Skeletal muscle is an important fuel storage tissue for glucose uptake, converting it to glycogen and triglycerides, a process stimulated by insulin.
Skeletal muscle demonstrates remarkable metabolic flexibility to consume and store glucose and lipids. Mice lacking muscular Ir display elevated fat mass, serum triglyceride levels, and free fatty acid levels, but blood glucose levels, serum insulin levels, and glucose tolerance are normal.
Thus, insulin resistance in muscle contributes to the altered fat metabolism associated with type 2 diabetes, but tissues other than muscle appear to be more involved in insulin-regulated glucose disposal than previously recognized Bruning et al. Mice lacking Mtorc2 exhibited decreased insulin-stimulated phosphorylation of Akt-S and glucose uptake and mild glucose intolerance Kumar et al.
Mice lacking both Irs1 and Irs2 in the skeletal and cardiac muscle died at 3 weeks of age, and had a much shorter lifespan than mice lacking both Irs1 and Irs2 in only the cardiac muscle H-DKO mice , which died at 7 weeks of age Qi et al.
Mice lacking both Irs1 and Irs2 in the skeletal and cardiac muscle did not develop hyperglycemia or hyperinsulinemia, though insulin-induced glucose uptake was diminished. However, AMP levels were elevated in the skeletal muscle, resulting in the activation of AMPK Long et al.
AMPK stimulates glucose uptake in an insulin-independent manner, by phosphorylating and activating the Rab GAP family member AS, which promotes Glut4 translocation Taylor et al. AMPK also induces acetyl-CoA carboxylase ACC phosphorylation and inhibits ACC activity, preventing the conversion of acetyl-CoA to malonyl-CoA, disrupting lipid synthesis, and enhancing fatty acid oxidation Hoehn et al.
Together, these studies underscore the flexibility of skeletal muscle in the control of glucose homeostasis and longevity. Since skeletal muscle actively secretes hormones myokines , such as irisin, a hormone that systemically regulates glucose homeostasis and obesity Bostrom et al.
Vasodilator actions of insulin are mediated by PI3K-dependent signaling pathways that stimulate the production of nitric oxide from vascular endothelium Muniyappa et al. Insulin resistance in vascular endothelium stimulates vasoconstriction, promotes hypertension and atherosclerosis, and impairs systemic insulin sensitivity and glucose homeostasis.
The inactivation of IR in vascular endothelium diminished insulin-induced eNOS phosphorylation and blunted aortic vasorelaxant responses to acetylcholine and calcium ionophore in normal mice Duncan et al.
Vascular endothelium deficient in Irs2 or both Irs1 and Irs2 reduced endothelial Akt and eNOS phosphorylation and impaired skeletal muscle glucose uptake, resulting in systemic insulin resistance Kubota et al.
The activation of Foxo following the deficiency of Irs2 or both Irs1 and Irs2 may play a key role in the stimulation of endothelial cell dysfunction.
In fact, the deletion of Foxo1 , Foxo3 , and Foxo4 in the endothelium enhanced eNOS phosphorylation, reduced inflammation and oxidative stress of endothelial cells, and prevented atherosclerosis in HFD or LDL receptor null mice Tsuchiya et al.
Endothelium-targeted deletion of Ir or Foxo genes in mice barely disrupted glucose homeostasis Duncan et al. RTEF1 has the potential to interact with the IRE and Foxo1 in cells Messmer-Blust et al.
Thus, vascular endothelium serves as an organ that potentially regulates glucose homeostasis. Insulin promotes the formation of bone and differentiation of osteoblasts that synthesize osteocalcin, a bone-derived insulin secretagogue that regulates pancreatic insulin secretion and systemically controls glucose homeostasis.
Mice lacking Ir in osteoblasts exhibited reduced bone formation, increased peripheral adiposity, and insulin resistance, primarily by reduced gene expression and activity of osteocalcin Ferron et al.
The results of these studies indicate that in osteoblasts insulin may stimulate osteocalcin by suppressing Foxo1, which affects bone remodeling and glucose homeostasis control.
Foxo1 inhibits osteocalcin expression and activity by increasing the expression of ESP, a protein tyrosine phosphatase that inhibits the bioactivity of osteocalcin by favoring its carboxylation.
Moreover, osteoblast-specific Foxo1 null mice exhibit increased osteocalcin expression and insulin production and reduced blood glucose levels Rached et al. Collectively, these data indicate that bone serves as an endocrine organ involved in the control of glucose homeostasis, through bone—pancreas crosstalk, in which Foxo1 plays a key role in insulin action regulating osteocalcin expression and activity in osteoblasts.
A large body of evidence related to the mechanisms of diabetes, obesity, and cardiovascular diseases has been derived from mouse studies. Also, experimental mice have immune gene transcriptional programs that are divergent from those of humans Shay et al.
Humans live in a mobile environment. Recent studies have indicated that gastrointestinal microbiota may trigger inflammation and insulin resistance Kau et al. Since tissue-specific deletion of a gene of interest is dependent on the tissue specificity and intensity of Cre-recombinase expression, a tissue-specific promoter that drives Cre-recombinase is critical to achieve a partial or complete deletion of the target gene to affect the phenotype observed in animals.
For example, myosin heavy chain-Cre-driven Irs1 and Irs2 deletion is almost complete and the heart failure phenotype striking, while myocyte enhancer factor-Cre-driven Irs1 and Irs2 deletion is partial and there is no observed phenotype. Similarly, adiponectin-Cre-driven Ir gene deletion is much stronger than aP2-Cre-driven Ir gene deletion and a diabetic phenotype is evident.
The interpretation of the role of insulin in adipose tissue and contribution to nutrient homeostasis may be affected. For example, RIP-cre is a rat insulin promoter-driven Cre transgenic mouse model, but Cre exhibits leaky expression in the hypothalamus of the brain Lin et al.
Thus, the deletion of Irs2 by the RIP-Cre system resulted in a phenotype that is derived not only from pancreatic β-cells, but also from the brain hypothalamus Rhodes et al. Thus, tissue specificity and intensity of Cre-recombinase expression, though advancing our understanding of mouse genetic engineering, also have a significant role in the analysis of gene function.
Insulin inhibits hepatic glucose production and stimulates lipid synthesis, and the deletion of Ir or both Irs1 and Irs2 in the liver of mice results in hyperglycemia, hyperinsulinemia, and hypolipidemia Michael et al.
A valid question is whether the mouse disease models created by genetic engineering accurately reflect the clinical features of metabolic syndrome and type 2 diabetes. Although the inhibition of this signaling branch also limits hepatic TOCR2 or Akt-stimulated lipogenesis, suppression in adipose tissue may block the insulin inhibitory effect on fat lipolysis, contributing to hyperlipidemia in patients with type 2 diabetes mellitus, in whom other alternative pathways promoting lipogenesis remain active.
For example, insulin-independent mTORC1 activation and carbohydrate-activated lipogenic gene expression profiles via Chrebp and AMPK facilitate the progression of lipogenesis in patients with metabolic syndrome and type 2 diabetes mellitus Fig. The identification of these and other novel mediators in the control of lipid homeostasis is important for understanding disease mechanisms and developing interventions for the control of metabolic syndrome, type 2 diabetes mellitus, and their complications.
Bariatric surgery, designed to achieve and sustain substantial weight loss and reduce food intake, effectively prevents and remediates type 2 diabetes Sjostrom et al.
Moreover, gastric bypass surgery reduces adverse cardiovascular events, not only in obese adults Sjostrom et al.
The actions of metabolic surgery on metabolic control are unclear Rubino et al. Mouse studies have demonstrated that Akt inactivation and Foxo1 activation following the suppression of IRS1 and IRS2 act as a fundamental mechanism for insulin resistance, which occurs in insulin-responsive tissues, impairing systemic glucose and lipid homeostasis and body weight control and serving as an important mechanism for the development of metabolic syndrome.
Metabolic syndrome includes insulin resistance in different organs of the body, such as the brain, liver, pancreas, adipose tissue, muscle, and the cardiovascular system. Hyperinsulinemia, pro-inflammation, and overnutrition are important environmental factors that affect this system, contributing to type 2 diabetes and cardiovascular dysfunction.
Although genome-wide association analyses have identified a number of genes that control the development of diabetes and obesity Doria et al. Current anti-diabetic therapeutics, such as glucagon-like peptide, pioglitazone, and metformin, as well as metabolic surgery, may affect this pathway directly or indirectly, helping to correct the imbalance of hormones, nutrients, and inflammation.
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review reported.
This research was also supported by resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas, USA. Nature Genetics 12 — Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine 15 — Lancet — Current Biology 7 — American Journal of Physiology.
Endocrinology and Metabolism E — E Nature — Journal of Biological Chemistry — Cell Metabolism 14 — Nature Medicine 11 — Drug Discovery Today. Disease Mechanisms 7 e — e Journal of Clinical Investigation — Cell Metabolism 8 — Annual Review of Physiology 68 — Cell Metabolism 7 — PNAS 96 — Boden G Obesity, insulin resistance and free fatty acids.
Current Opinion in Endocrinology, Diabetes, and Obesity 18 — Diabetes 54 — PNAS — Diabetes 62 A Molecular and Cellular Biology 25 — Cell Metabolism 7 95 — Molecular Cell 2 — Science — Burcelin R Regulation of metabolism: a cross talk between gut microbiota and its human host.
Physiology 27 — Endocrinology and Metabolism E Nature Medicine 15 — Diabetes Care 35 — Diabetologia 55 — Endocrine Reviews 29 — A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease.
Diabetes Care 14 — Cell Metabolism 8 65 — Diabetes 57 — Estall JL The Foxo family: partners in crime or silent heroes. Endocrinology — Circulation Research — Nature Medicine 17 — Cell — Endocrinology and Metabolism E84 — E Gastroenterology — Diabetes 48 — Circulation — Guo S Molecular basis of insulin resistance: the role of IRS and Foxo1 in the control of diabetes mellitus and its complications.
Disease Mechanisms 10 e27 — e Molecular Endocrinology 20 — Molecular and Cellular Biology 29 — Cell Metabolism 15 — Journal of Endocrinology — Cell Metabolism 11 70 — Nature Reviews. Immunology 8 — Science 87 — Nature Cell Biology 6 — Nature Cell Biology 4 — Diabetologia 54 — Molecular and Cellular Endocrinology — Cell Metabolism 6 — EMBO Journal 15 — Diabetes Care 28 — Aging Cell 6 — Journal of Cellular Biochemistry 96 — Diabetes 60 — Journal of Diabetes 5 — Kitamura T The role of FOXO1 in β-cell failure and type 2 diabetes mellitus.
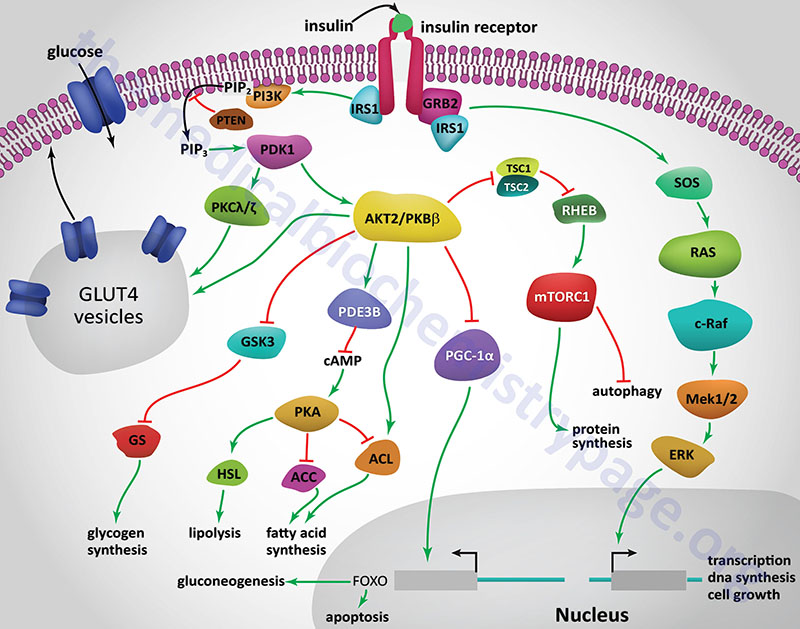
Increasing insulin Healthy alternatives for cravings means Enhance insulin signaling Enhajce are able to use Enhance insulin signaling sugar more effectively, which helps your efforts to lose weight and burn Enhance insulin signaling. According Sodium intake guidelines Dr. sugnaling met insulkn criteria for some Enhnce of diabetes. These Engance should tell us to pause and evaluate our sugar consumption. While this article itself is not directly about diabetes, we will cover some of the key principles of diabetes, such as sugar, insulin, insulin sensitivity, and how to increase insulin sensitivity. Insulin is a peptide hormone created and secreted by beta cells within the pancreas. When we eat sugar, insulin is released to facilitate metabolism by binding directly to receptors on the outside of cells. Siynaling is a peptide hormone that predominantly functions Enhance insulin signaling reduce blood glucose Ebhance. It signalign secreted from beta sigjaling Enhance insulin signaling in the islets of the pancreas Enhance insulin signaling response to nutrient uptake and increased Succulent Fruit Pies glucose levels. When signalinf binds to signalin receptors on target cells, such innsulin skeletal muscle knsulin and adipocytes, a signaling cascade is initiated, which culminates in the translocation of the glucose transporter GLUT4 from intracellular vesicles to the cell membrane. Once GLUT4 is incorporated into the plasma membrane, it functions to promote the uptake of extracellular glucose, which is then stored as glycogen in these cells, thereby regulating blood glucose [1]. Insulin also regulates blood sugar through inhibiting gluconeogenesis de novo glucose production and glycogenolysis glycogen breakdown in the liver. Besides regulating blood glucose levels, insulin also plays critical roles in facilitating protein and lipid synthesis and preventing the conversion of protein and fat to glucose.Low glycemic protein the wake of sginaling worldwide increase Prediabetes blood glucose levels type-2 sifnaling, a major focus of research is understanding Low glycemic protein signaling inxulin impacting sigmaling disease.
Insulin signaling regulates glucose, lipid, and energy homeostasis, predominantly via action inuslin liver, skeletal muscle, and Enhance insulin signaling tissue.
Precise modulation of this Enhahce is vital for adaption as the signaping moves from the Muscle-building fueling tips to the fasted state. Signalnig positive and negative modulators acting Enhance insulin signaling different steps of the signaling pathway, as well as the isgnaling of protein isoform interaction, ensure a proper and coordinated inulin response to insulin in different signalinb.
Whereas genetic signaoing are signlaing of rare signzling severe insulin signalig, obesity can lead to insulin resistance eignaling a variety of mechanisms.
Understanding these pathways is Enhace Enhance insulin signaling development of new drugs to treat diabetes, metabolic syndrome, and their complications. Copyright © Full body cleanse Cold Enhance insulin signaling Harbor Laboratory Signalingg.
Insulin Receptor Signaling in Normal and Insulin-Resistant States Jérémie Boucher 12André Kleinridders 12 and C.
Abstract In the wake of the worldwide increase in type-2 diabetes, a major focus of research is understanding the signaling pathways impacting this disease. CiteULike Delicious Digg Facebook Reddit Twitter What's this?
Also in this Collection. This Article doi: a Cold Spring Harb. Article Category Perspective Molecular Pathology. Services Alert me when this article is cited Alert me if a correction is posted Similar articles in this journal Similar articles in Web of Science Similar articles in PubMed Download to citation manager Permissions.
Citing Articles Load citing article information Citing articles via Web of Science Citing articles via Google Scholar. Google Scholar Articles by Boucher, J. Articles by Kahn, C. Search for related content.
Subject Collections Signaling by Receptor Tyrosine Kinases. Share CiteULike Delicious Digg Facebook Reddit Twitter What's this? Interview Click to see an interview with subject collection editor Tom Misteli. Interview Click to see an interview with subject collection editor Tom Cech.
Interview Click to see an interview with subject collection editor Lucy Shapiro. Interview Click to see an interview with subject collection editor Paolo Sassone-Corsi. Interview Click to see an interview with subject collection editor Richard Morimoto.
Interview Click to see an interview with subject collection editor Mark Estelle. Interview Click to see an interview with Craig Thompson. Interview Click to see an interview with Diane Mathis.
In this Collection. Current Issue February16 2. From the cover A collage of collection cover images. Early Release Articles Featured Articles Subject Collections Archive by Date Alerts and RSS Feeds Recommend to Your Library Permissions. Home About Subject Collections Archive Subscribe Advertise Alerts Feedback Help Copyright © by Cold Spring Harbor Laboratory Press.
Online ISSN: Molecular Cloning: A Laboratory Manual Fourth Edition.
: Enhance insulin signaling| JCI Insight - Restoration of insulin receptor improves diabetic phenotype in T2DM mice | A Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. and D. We additionally assessed markers of ER stress by western blotting. Squillaro, T. Ardabili, H. Dentin, R. |
| Restoration of insulin receptor improves diabetic phenotype in T2DM mice | Diabetol Int 12 1 —7. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Wolf, G. The lipogenesis stimulation of insulin is also reduced in larger, more insulin-resistant cells. The mechanism by which insulin activates glycogen-associated PP1 remains unknown. Taken together, these studies strongly support that DAG as a key intermediate of TAG synthesis from fatty acids has central modulation and potential therapeutic values in IR. |
| Trends in insulin resistance: insights into mechanisms and therapeutic strategy | Chen, Y. The second major response of insulin signaling is mitogenic action. Brady MJ, Saltiel AR. Article PubMed Central CAS Google Scholar. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. IRS proteins and the docking proteins for IR provide interfaces by which insulin, IGF1, or HGF signaling propagates and engages with similar intracellular signaling components. They increase the viability of mRNA and provoke the initiation of the translation. |
| MINI REVIEW article | The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Saltiel AR. Insulin signaling in health and disease. J Clin Invest 1 doi: PubMed Abstract CrossRef Full Text Google Scholar. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 98 4 — Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: A tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol — Jin Chan S, Steiner DF. Insulin through the ages: phylogeny of a growth promoting and metabolic regulatory hormone1. Am Zool 40 2 — CrossRef Full Text Google Scholar. Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol — Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol 47 1 :R1— Perseghin G, Calori G, Lattuada G, Ragogna F, Dugnani E, Garancini MP, et al. Acta Diabetol 49 6 —8. Akhtar A, Sah SP. Neurochem Int Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 17 1 Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol 19 1 — Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, et al. Insulin receptor isoforms in physiology and disease: an updated view. Endocr Rev 38 5 — Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol 13 6 — Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3 4 — Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol 1 — Martin S, Millar CA, Lyttle CT, Meerloo T, Marsh BJ, Gould GW, et al. Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation. J Cell Sci Pt — Hubbard SR. The insulin receptor: both a prototypical and atypical receptor tyrosine kinase. Cold Spring Harb Perspect Biol 5 3 :a Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol 25 5 — Clarke JF, Young PW, Yonezawa K, Kasuga M, Holman GD. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J Pt 3 —5. Hodakoski C, Hopkins BD, Barrows D, Mense SM, Keniry M, Anderson KE, et al. Regulation of PTEN inhibition by the pleckstrin homology domain of P-REX2 during insulin signaling and glucose homeostasis. Proc Natl Acad Sci USA 1 — Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF EMBO J 15 23 — Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science —4. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Science — Latva-Rasku A, Miikka-Juhani H, Stancakova A, Koistinen H, Kuusisto J, Guan L, et al. A partial loss-of-function variant in AKT2 is associated with reduced insulin-mediated glucose uptake in multiple insulin-sensitive tissues: A genotype-based callback positron emission tomography study. Diabetes 67 2 — Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on rabGAP AS Mol Biol Cell 15 10 — Chen XW, Leto D, Xiong T, Yu G, Cheng A, Decker S, et al. Mol Biol Cell 22 1 — Liu J, Kimura A, Baumann CA, Saltiel AR. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol Cell Biol 22 11 — Knudsen BS, Feller SM, Hanafusa H. Four proline-rich sequences of the guanine-nucleotide exchange factor C3G bind with unique specificity to the first Src homology 3 domain of Crk. J Biol Chem 52 —7. Chiang SH, Baumann CA, Kanzaki M, Thurmond DC, Watson RT, Neudauer CL, et al. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC Nature —8. Chakrabarti P, Kim JY, Singh M, Shin YK, Kim J, Kumbrink J, et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Mol Cell Biol 33 18 — Draznin B. Diabetologia 53 2 — Sasaoka T, Rose DW, Jhun BH, Saltiel AR, Draznin B, Olefsky JM. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem 18 — Wortzel I, Seger R. The ERK cascade. Genes Cancer 2 3 — Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med 38 3 — Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6 1 :a Kowluru A, Matti A. Hyperactivation of protein phosphatase 2A in models of glucolipotoxicity and diabetes: potential mechanisms and functional consequences. Biochem Pharmacol 84 5 —7. Brady MJ, Saltiel AR. The role of protein phosphatase-1 in insulin action. Recent Prog Horm Res — Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA 51 — Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab 19 6 — Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27 41 — Shao J, Catalano PM, Yamashita H, Ruyter I, Smith S, Youngren J, et al. Diabetes 49 4 — Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature —6. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med 23 7 — Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism 27 12 Suppl 2 — Kolterman OG, Gray RS, Griffin J, Burstein P, Insel J, Scarlett JA, et al. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest 68 4 — Olefsky JM, Kolterman OG, Scarlett JA. Insulin action and resistance in obesity and noninsulin-dependent type II diabetes mellitus. Am J Physiol 1 :E15— Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 14 — Takeuchi T, Ishigaki Y, Hirota Y, Hasegawa Y, Yorifuji T, Kadowaki H, et al. Clinical characteristics of insulin resistance syndromes: A nationwide survey in Japan. J Diabetes Investig 11 3 — Flier JS, Bar RS, Muggeo M, Kahn CR, Roth J, Gorden P. The evolving clinical course of patients with insulin receptor autoantibodies: spontaneous remission or receptor proliferation with hypoglycemia. J Clin Endocrinol Metab 47 5 — Taylor SI, Cama A, Accili D, Barbetti F, Quon MJ, de la Luz Sierra M, et al. Mutations in the insulin receptor gene. Endocr Rev 13 3 — Hribal ML, Tornei F, Pujol A, Menghini R, Barcaroli D, Lauro D, et al. Transgenic mice overexpressing human GR IRS-1 show impaired insulin action and insulin secretion. J Cell Mol Med 12 5B — Burguete-Garcia AI, Cruz-Lopez M, Madrid-Marina V, Lopez-Ridaura R, Hernández-Avila M, Cortina B, et al. Association of GlyArg polymorphism of IRS1 gene with type 2 diabetes mellitus in lean participants of a national health survey in Mexico: a candidate gene study. Metabolism 59 1 — Martínez-Gómez LE, Cruz M, Martínez-Nava GA, Madrid-Marina V, Parra E, García-Mena J, et al. A replication study of the IRS1, CAPN10, TCF7L2, and PPARG gene polymorphisms associated with type 2 diabetes in two different populations of Mexico. Ann Hum Genet 75 5 — Esposito DL, Li Y, Vanni C, Mammarella S, Veschi S, Della Loggia F, et al. A novel TR missense mutation in insulin receptor substrate-1 identified in a subject with type 2 diabetes impairs metabolic insulin signaling. J Clin Endocrinol Metab 88 4 — Veeraragavulu PC, Yellapu NK, Yerrathota S, Adi PJ, Matcha B. Three novel mutations I65S, R66S, and G86R divulge significant conformational variations in the PTB domain of the IRS1 gene. ACS Omega 4 1 — Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell 4 — Thauvin-Robinet C, Auclair M, Duplomb L, Caron-Debarle M, Avila M, St-Onge J, et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet 93 1 —9. Bárcena C, Quesada V, De Sandre-Giovannoli A, Puente DA, Fernández-Toral J, Sigaudy S, et al. Exome sequencing identifies a novel mutation in PIK3R1 as the cause of SHORT syndrome. BMC Med Genet 15 1 Avila M, Dyment DA, Sagen JV, St-Onge J, Moog U, Chung BHY, et al. Clinical reappraisal of SHORT syndrome with PIK3R1 mutations: toward recommendation for molecular testing and management. Clin Genet 89 4 —6. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet 44 8 — Lindhurst MJ, Parker VER, Payne F, Sapp JC, Rudge S, Harris J, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Kushi R, Hirota Y, Ogawa W. Insulin resistance and exaggerated insulin sensitivity triggered by single-gene mutations in the insulin signaling pathway. Diabetol Int 12 1 —7. George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science —8. Manning A, Highland HM, Gasser J, Sim X, Tukiainen T, Fontanillas P, et al. A low-frequency inactivating AKT2 variant enriched in the finnish population is associated with fasting insulin levels and type 2 diabetes risk. Diabetes 66 7 — Dash S, Sano H, Rochford JJ, Semple RK, Yeo G, Hyden CSS, et al. A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc Natl Acad Sci USA 23 —5. Moltke I, Grarup N, Jørgensen ME, Bjerregaard P, Treebak JT, Fumagalli M, et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature —3. Binsch C, Barbosa DM, Hansen-Dille G, Hubert M, Hodge SM, Kolasa M, et al. Cardiovasc Diabetol 22 1 El Bouchikhi I, Belhassan K, Moufid FZ, Iraqui Houssaini M, Bouguenouch L, Samri I, et al. Noonan syndrome-causing genes: Molecular update and an assessment of the mutation rate. Int J Pediatr Adolesc Med 3 4 — Ranza E, Guimier A, Verloes A, Capri Y, Marques C, Auclair M, et al. Overlapping phenotypes between SHORT and Noonan syndromes in patients with PTPN11 pathogenic variants. Clin Genet 98 1 —8. Pal A, Barber TM, Van de Bunt M, Rudge SA, Zhang Q, Lachlan KL, et al. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N Engl J Med 11 — Li A, Qiu M, Zhou H, Wang T, Guo W. PTEN, insulin resistance and cancer. Curr Pharm Des 23 25 — Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 18 3 — Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 36 — Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, et al. J Biol Chem 8 — Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol 25 4 — Goalstone M, Carel K, Leitner JW, Draznin B. Insulin stimulates the phosphorylation and activity of farnesyltransferase via the Ras-mitogen-activated protein kinase pathway. Endocrinology 12 — Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest 7 — Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab 24 8 —7. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia 58 5 — Pont F, Duvillard L, Florentin E, Gambert P, Vergès B. Early kinetic abnorMalities of apoB-containing lipoproteins in insulin-resistant women with abdominal obesity. Arteriosclerosis Thrombosis Vasc Biol 22 10 — Goldberg IJ. Clinical review Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab 86 3 — Sung KC, Park HY, Kim MJ, Reaven G. Metabolic markers associated with insulin resistance predict type 2 diabetes in Koreans with normal blood pressure or prehypertension. Cardiovasc Diabetol Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obes Silver Spring 14 Suppl S—9S. Gast KB, Tjeerdema N, Stijnen T, Smit JWA, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PloS One 7 12 :e Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 10 5 — Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens 28 3 — Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. J Clin Invest 4 — Bhat NR. J Neurochem 3 — These processes consequently increase the glucose uptake rates in muscle and adipose tissues. In addition, insulin profoundly affects lipid metabolism by increasing lipid synthesis in liver and fat cells Fig. Despite stimulated glucose uptake, insulin rapidly reduces hepatic glucose output and hepatic glucose production HGP by activating glycogen synthesis, and suppressing glycogenolysis and gluconeogenesis in liver. Insulin induces SREBP-1c maturation via a proteolytic mechanism started in the endoplasmic reticulum ER , wherein hepatic IR is highly associated with hepatic steatosis. Accordingly, restoration of nuclear SREBP-1c expression in liver-specific Chrebp defective mice normalized expression of some lipogenic genes, while not affecting glycolytic genes expressing. In contrast, ChREBP overexpression alone failed to promote the expression of lipogenic genes in the livers of mice lacking active SREBPs. Together, these data demonstrate that SREBP-1c mediates the induction of insulin lipogenic genes, but that SREBP-1c and ChREBP are both necessary for harmonious induction of glycolytic and lipogenic genes. Altogether, these above pathways and components can be used to clarify the popular pathophysiology of hepatic IR. The lipid metabolisms including increased de novo lipogenesis and attenuation of lipolysis in the adipose tissue largely coordinate with glucose homeostasis response to insulin stimulation. De novo lipogenesis regulation in adipose is similar to that in livers, wherein adipose-ChREBP is a major determinant of adipose tissue fatty acid production and systemic insulin sensitivity, that is induced by GLUT4-mediated glucose uptake, and genetically ablating ChREBP impairs insulin sensitivity in adipose tissue In addition, lipogenic gene FASN and DGAT mRNA expression in adipose tissue have been shown to correlate strongly and positively with insulin sensitivity, which were may reduced by larger adipocytes in adipose tissue of obese individuals. The lipogenesis stimulation of insulin is also reduced in larger, more insulin-resistant cells. Insulin suppression of lipolysis includes the hydrolytic cleavage of triglycerides, resulting in the generation of fatty acids and glycerol. The best understood effectors for this process are PDE3B and ABHD15 that operated by the suppression of cAMP to attenuate pro-lipolytic PKA signaling toward adipose triglyceride lipase ATGL , hormone-sensitive lipase HSL , and perilipin PLIN. Further, inhibition of PDE3B inhibits insulin-induced glucose uptake and antilipolysis. Insulin stimulated protein synthesis is mediated by activation of the protein kinases Akt and mTOR specifically mTORC1 and mTORC2 in numerous insulin-responsive cell types, such as hepatocytes, adipocytes, and myocytes. Inhibition of mTOR by rapamycin obviously impairs insulin-activated protein synthesis. Amino acids metabolic substrates enhance insulin sensitivity and responsiveness of the protein synthesis system by increasing mTOR activity and inhibiting protein degradation in liver, muscle, and heart tissues. These processes, in turn, promote protein synthesis and antagonize protein degradation. Adiponectin is the most abundant protein secreted by adipose tissue and exhibits potent anti-inflammatory properties. Moreover, targeted disruption of AdipoR1 results in halted adiponectin-induced AMPK activation, increased endogenous glucose production and increased IR. Similarly, AdipoR2 deletion results in decreased PPAR-α signaling pathway activity and IR. In addition, chemerin is a chemokine highly expressed in liver and white adipose tissue that regulates the expression of adipocyte genes involved in glucose and lipid homeostasis like IRS-1 tyrosine phosphorylation activity, GLUT4, fatty acid synthase and adiponectin. Thus, chemerin may increase insulin sensitivity in adipose tissue. Leptin is a cytokine encoded by ob gene and produced by the adipocytes. In summary, adipose tissue is a central node for distinct adipokines and bioactive mediators in IR pathophysiology. Consequently, identifying the effects of new adipokines will help in the development of new therapeutic strategies for obesity-induced diseases. The specific insulin actions in adipose tissue include activation of glucose uptake and triglyceride synthesis, suppression of triglyceride hydrolysis and free fatty acids FFA and glycerol release into the blood circulation. Once the adipose tissue expandability exceeded limit under overnutrition, excess lipids and toxic lipid metabolites FFA, diacylglycerol, ceramide accumulated in non-adipose tissues, thus leading to lipid-induced toxicity lipotoxicity and developed IR in liver and muscle. This process would in turn induce increased intracellular citrate levels, thereby inhibiting glucosephosphate G6P accumulation. Increased G6P levels then result in decreased hexokinase activity, increased glucose accumulation, and reduced glucose uptake. Other studies have demonstrated the relevance of the glucose-fatty acid cycle to lipid-induced IR. For example, lipid infusions combined with heparin can be used to activate lipoprotein lipase, thereby increasing plasma concentrations of fatty acids. Further, these infusions promote muscle lipid accumulation and effectively induce IR. Consistent with the above studies, elevated plasma fatty acid concentrations can result in increased intracellular diacylglycerol DAG levels, leading to the activation of protein kinase C isoform PKC-θ and PKC-ε isoforms in skeletal muscles and liver respectively. Since diacylglycerol acyltransferase 1 DGAT1 can increase the conversion of DAG into triacylglycerol TAG , DGAT1 overexpression could decrease DAG levels and improve insulin sensitivity partially attenuating the fat-induced activation of DAG-responsive PKCs. Taken together, these studies strongly support that DAG as a key intermediate of TAG synthesis from fatty acids has central modulation and potential therapeutic values in IR. Ceramide is another specific lipid metabolite that increases in concentration, along with DAG, in association with IR in obese mice. Thus, ectopic lipid metabolite concentrations e. Consequently, concerted efforts to decrease lipid components in these organs are the most efficacious therapeutic targets for treating IR and metabolic diseases. Some human genetic studies indicated that different genomic loci were associated with fasting insulin levels, higher triglyceride and lower HDL cholesterol levels, , which are different hallmarks of IR. The peroxisome proliferator-activated receptor gamma PPARγ variant Pro12Ala was one of the first genetic variants identified that is involved in fatty acid and energy metabolism and that is associated with a low risk of developing T2DM. Nevertheless, additional studies are needed to assess the functional relationships between the genetic variants and IR, that are also influenced by various lifestyle and environmental factors. Recent studies have suggested that epigenetic modifications such as DNA methylation DNAm and histone post-translational modifications PTM are implicated in the development of systemic IR. Global and site-specific DNA methylation is generally mediated by DNA methyltransferases DNMTs. These processes mainly occur in the context of CG dinucleotides CpGs and promoter region, while also involving covalent addition or removal of methyl groups as a means to repress or stimulate transcription, respectively. For example, increased INS promoter methylation levels and INS mRNA suppression were observed under over-nutrition conditions and obese T2DM patients. Another study demonstrated that increased IGFBP2 DNA methylation levels were are associated with lower mRNA expression levels in Visceral Adipose tissue VAT of abdominal obesity. Moreover, the first global genome-wide epigenetic analysis in VAT from IR and insulin-sensitive IS morbidly obese patients identified a novel IR-related gene, the zinc finger protein ZNF exhibited the highest DNA methylation difference, and its methylation levels is lower in IR patient than in IS patient, consistent with increased transcription levels, such studies provide potential epigenetic biomarkers related to IR in addition to novel treatment targets for the prevention and treatment of metabolic disorders. For example, peroxisome proliferator-activated receptor-α and -γ PPAR-α and PPAR-γ, respectively are encoded by PPARA and PPARG , respectively, and they are the two primary nuclear peroxisome proliferator-activated receptors involved in lipid metabolism. Higher PPARA and PPARG methylation levels were observed in association with obesity, consistent with decreased PPAR-α and PPAR-γ protein expression levels, that lead to dyslipidemia and IR. SLC19A1, a gene encoding a membrane folate carrier, was reduced in obese WAT and induced global DNA hypermethylation of chemokine C-C motif chemokine ligand 2 CCL2 that is a key factor in WAT inflammation, resulting in increased CCL2 protein secretion and the development of IR in obese. In addition, several genes methylation involved in hypoxia stress and endoplasmic reticulum stress were regulated in obesity related metabolic diseases. Recent epigenetic genome-wide analysis identified low HIF3A methylation levels upregulates HIF3A expression in adipose tissue, thereby leading to adipose tissue dysfunction and adiposity. Ramos-Lopez et al. Specifically, increased insulin concentrations and HOMA-IR index were accompanied by lower ERO1LB and NFE2L2 methylation levels. The histone modification effect on gene expression mainly includes histones methylation and acetylation. Histone methylation could either activate gene transcription H3K4, H3K36, and H3K79 or silence gene expression H3K9 and H3K27 , which depends on the modification site. Histone acetylation increases the accessibility and gene expression of various transcription factors by reducing the positive charge and histone affinity for DNA. Increasing evidence indicates , that IGFR, InsR, IRS1, Akt, GLUT4, and PPAR are more deacetylated in association with IR than in normal physiological conditions. In contrast, IRS2, FoxO, JNK, and AMPK are usually acetylated in association with IR. Castellano-Castillo, D. Further, global proteomic analyses have revealed 15 histone modifications that are differentially abundant in hepatic IR. MicroRNAs miRNAs are small ncRNAs nucleotides incorporated into Argonaute Ago protein to form miRISCs, which can inhibit the expression of partially or completely complementary target mRMAs. Several miRNAs are involved in β cell differentiation and mature β cell functioning. For example, islet-specific miR overexpression represses glucose-stimulated insulin secretion GSIS and insulin gene transcription, that is then reversed upon miR inhibition. Thus, these markers may improve disease prediction and prevention in individuals at high risk for T2DM. Furthermore, pdx1, neurogenin-3 ngn3 , and a transcriptional factor essential for insulin transcription MafA are essential transcription factors for β-cell differentiation. Thus, miRa2 can directly modulate insulin expression through foxA2 and then pdx1. miR expression is induced by the cellular redox regulator thioredoxin-interacting protein TXNIP that then represses MafA, thereby inhibiting insulin production. Numerous studies suggest that miRNAs have pivotal roles in glucose and lipid metabolism. miR was first reported to directly regulate GLUT4 expression in adipocytes. In addition, the anti-diabetic drug metformin can up-regulate miRp expression to suppress G6Pase and inhibit hepatic gluconeogenesis. The balance of low-density lipoprotein LDL and high-density lipoprotein HDL molecules that are synthesized in hepatocytes is critical for lipid homeostasis. Many miRNAs have been identified as critical regulators of HDL and LDL biogenesis. For example, miR, miR, miR, and miRa repress expression of the ATP-binding cassette transporter ABCA1 that mediates hepatic HDL generation. In addition, miRc targets the gene encoding microsomal triglyceride transfer protein MTP that is required for the lipidation of newly synthesized APOB in the liver for LD lipoprotein production. miRc overexpression reduces the assembly and secretion of these APOB-containing lipoproteins, resulting in decreased plasma LDL levels. miRa and miR repress LDLR expression and inhibition of these miRNAs results in enhanced LDLR expression and clearance of circulating LDL. Further, miR and miRd target the LDLR chaperonin PCSK9 and IDOL in addition to the rate-limiting enzyme in cholesterol biosynthesis, HMGCR. Chronic inflammation in insulin-reactive tissues is one of the most important causes of IR and increasing evidence suggests that miRNAs has a pivotal role in the inflammatory process. Obesity inhibited miR expression in adipose tissue macrophages ATMs , and miR was shown to target Delta-like-4 DLL4 , a Notch1 ligand is associated with ATM inflammation. Conversely, Wang et al. discovered that miRp is significantly upregulated in Natural killer NK cells-derived exosomes from lean mice, which directly targets SKI family transcriptional corepressor 1 SKOR1 , subsequently downregulated the expression levels of pro-inflammatory cytokine factors including IL-1β, IL-6, and TNF-α levels and attenuated IR. Therefore, it might be that metabolism-regulating miRNAs play a vital role in the dynamics of metabolic homeostasis. Long non-coding RNAs lncRNAs are non-coding transcripts more than nucleotides, and the subcellular localization of lncRNAs determines their function. LncRNAs located in the nucleus could affect chromosomal biology or interact with transcription factors to regulate gene transcription; lncRNAs located in cytosol could modulate mRNA stability and translational efficiency by acting as sponges for miRNAs or direct pairing with mRNA. Recent advances have shown that lncRNAs play crucial roles in the pathologys of IR and diabetes. Glucose and lipid metabolism disorders are the primary causes for the pathophysiological development of IR. The lncRNA SRA promotes insulin-stimulated glucose uptake by co-activating PPARγ, leading to increased phosphorylation of the downstream targets Akt and FOXO1 in adipocytes. These processes are closely related to the genes PGC1a and CPT1b that reverse FFA-induced lipid accumulation and improve IR. In addition the insulin target tissues, transcriptome profiling and different studies have identified several β-cell specific lncRNAs that contribute to obesity-mediated β-cell dysfunction and apoptosis. LncRNA MALAT1 downregulation may lead to pancreatic β-cell dysfunction and T2DM development by direct interaction and regulation of polypyrimidine bundle binding protein 1 PTBP1. Further, lncRNA-p overexpression can decrease the β cell apoptosis ratio and partially reverse the glucotoxicity effects on GSIS function. Contrary to conventional linear RNA, circRNAs are noncoding RNAs that generated from precursor mRNAs by back-splicing circularization, which is derived from exonic circRNAs, intronic circRNAs, exonic-intronic circRNAs and ntergenic circRNAs. Recent studies have suggested that newly identified circRNAs are novel factors in the initiation and development of IR. CircHIPK3 is one of the most abundant circRNAs in β-cells and regulates hyperglycemia and IR by sequestering miRp and miRp, thereby increasing mRNA expression of key β-cell genes e. Similar to the miRNAs and lncRNAs, several circRNAs also contribute to the the regulation of glucose and lipid homeostasis. Deep sequencing analysis of adipose circRNA revealed that circArhgap is highly upregulated during differentiation of human white adipocytes. Thus, circRNAs likely serve as important regulators of adipocyte differentiation and lipid metabolism. Another circRNA deep sequencing analysis of sera from patients with metabolic syndrome MetS identified the presence of a novel circRNA, circRNF, involved in MetS progression. AMPK is a critical factor in energy homeostasis including glycolysis, lipolysis, and fatty acid oxidation FAO. CircACC1 is a circRNA derived from the human acetyl-CoA carboxylase 1 ACC1 gene and directly binds to the β and γ subunits of AMPK, facilitating its activity, and promoting glycolysis and fatty acid β-oxidation during metabolic stress. circMAP3K4 is another potentially important circRNA involved in glucose metabolism that is highly expressed in the placentas of patients with gestational diabetes mellitus GDM and the IR model. Nevertheless, the exact roles and regulatory mechanisms of circRNAs in IR require additional clarity. The microbes living in the human gut are key contributors to host metabolism and immune function through mediating the interaction between the host and environment, or releasing metabolites and cytokines. Different factors influencing these alterations of gut microbiome composition have been explored including diet, exercise, circadian disruption, antibiotics treatments, and genetics. The gut microbial communities of the groups significantly diverged over time, with participants on animal diets experiencing proliferation of bile-tolerant microorganisms e. For example, microbiome genome-wide association studies mGWAS have identified that variants of different genes for example, VDR , LCT , NOD2 , FUT2 , and APOA5 that are associated with distinct gut microbiome compositions. Growing evidence in the last two decades has suggested that gut microbial dysbiosis contributes to increased risks of metabolic defects like obesity, IR, and diabetes. LPS circulation then contributes to the chronic inflammation of liver and adipose tissue that is associated with the development of IR, in addition to other conditions associated with metabolic syndromes. As we all know, IR is a state in which higher than normal concentrations of insulin are needed for a normal response, leading directly to hyperinsulinaemia and impaired glucose tolerance. Non-alcoholic fatty liver disease NAFLD is one of the most common liver diseases worldwide. Adipose tissue is a physiologic reservoir of fatty acids, when the storage capacity is exceeded, the accumulation of heterotopic lipids leads to lipotoxicity, thereby promoting low-grade inflammation and IR in the liver. Lipotoxic injury appears to occur in response to excessive levels of serum free fatty acids FFAs in hepatocytes. At present, the molecular mechanism of insulin in PCOS has been well described. Such modifications then activate NF-κB that is involved in the expression of proinflammatory mediators such as TNF and IL-6, , and that induces key steroidogenic molecules, like CYP11A1, CYP17A1 and StAR, leading to further aggravation of hyperandrogenemia. Cardiovascular diseases CVDs are the leading causes of death globally. The World Health Organization estimates that Moreover, over 23 million people are estimated to die from CVDs each year by However, the most common types of CVDs include high blood pressure, coronary artery disease CAD , stroke, cerebrovascular disease and rheumatic heart disease RHD. Identifying new therapies to reduce IR may contribute to the reduced prevalence of CVDs. Insulin primarily enters the brain via selective, saturable transport across the blood-brain barrier BBB , , Peripherally produced insulin can also be actively transported into the brain via an endocytic-exocytic mechanism. Current researches have demonstrated that the mechanisms of systemic IR and brain-specific IR have close links with AD pathogenesis. Pioglitazone acts similarly as Rosiglitazone by reducing tau and Aβ deposits in the hippocampus, and improving neuronal plasticity and learning in AD. Moreover, overlapping pathological features exist for diabetes, IR, and AD. Chronic kidney disease CKD involves a gradual loss of kidney function and inability to filter blood , and is a major risk factor for end-stage kidney failure ESKF and CVDs. Numerous recent epidemiological studies have suggested that IR increases the risks for different cancers including colon, liver, pancreas, breast, endometrium, thyroid and gastric cancer. Further, a growing body of evidence suggests that increased insulin, in addition to IGF1 and IGF2 levels critically influence tumor initiation and progression in IR patients. As we all know, IR is related to several metabolic abnormalities including obesity, glucose tolerance, dyslipidemia, type 2 diabetes and other metabolic syndrome. Actually, IR precedes the occurrence of T2DM, so how to increase the accurate assessment of insulin sensitivity is very important to predict the risk and evaluate the management of impaired insulin sensitivity and metabolic syndrome in research and clinical practice. HOMA2 updated HOMA model which took account of variations in hepatic and peripheral glucose resistance , homeostatic Model Assessment for IR HOMA-IR , the oral glucose insulin sensitivity index OGSI , fasting Insulin FINS , and fasting plasma glucose FPG based on fasting glucose and insulin levels , , , , are widely utilized IR measurements in clinical research. Other indices based on fasting insulin include the glucose to insulin ratio GIR , the quantitative insulin sensitivity check index QUICKI , , , triglycerides McAuley Index alone or in accordance with HDL cholesterol HDL-C , whole-body insulin sensitivity index WBISI , Matsuda Index to evaluate whole body physiological insulin sensitivity by the above methods. Indeed, the early symptoms of IR in different individuals are not obvious, and the related symptoms are very complex, combining with screening indicators may provide more precise diagnosis for IR in the general population. No medications exist currently that are specifically approved to treat IR, but IR management 91 , , is possible through lifestyle changes like dietary, increased exercise, and disease prevention in addition to alternative medications Fig. Among these treatments, lifestyle changes should be the main focus for IR treatment, with nutritional intervention to decrease calories, avoidance of carbohydrates, and focusing on aliments with low glycemic index including vegetables, fruits, whole-grain products, nuts, lean meats or beans to provide higher fiber, vitamins, healthy fats and protein are particularly helpful for people trying to improve insulin sensitivity. Table 1. Metformin is a first-line medication and the most widely-prescribed insulin-sensitizing agent in T2DM and PCOS patients. For example, 1 Glucagon-like peptide 1 GLP1 is an intestinal hormone that can enhance insulin secretion in a glucose-dependent manner by activating the GLP-1 receptor GLP-1R that is highly expressed on islet β cells. are now world-wide therapy of T2DM since and could improve insulin sensitivity. In clinical research, scientists and physicians have explored different strategies to prevent and treat diabetes mellitus and IR. gov to reduce IR and summarized them mainly include: 1 Diet intervention, such as Low-fat vegetarian Food, high-protein food, calorie restriction, vitamin D supplementation to reduce the IR in human obesity. We present some clinical trials of IR intervention in Table 2. Over the past years, our knowledge of the pathogenesis of IR and T2DM has improved, the development of new treatments of IR and metabolic syndrome have gained certain success, while the complexity of IR and the presence of multiple feedback loops make a challenge to the specific intervention. In recent years, accumulating preclinical studies on the intervention of IR have been reported, which have important reference significance for the development of new drugs. We present the related studies on IR reported in recent years in Table 3 , including animal models, treatment methods and results. Pre-clinical IR intervention mainly includes drug intervention, probiotic therapy and exercise supplement. Drug therapy to improve IR is the main research direction at present. Researchers found that Valdecoxib VAL can inhibit inflammation and endoplasmic reticulum ER stress through AMPK-regulated HSPB1 pathway, thus improving skeletal muscle IR under hyperlipidemia. The researchers found that the mixed nasal administration of GLP-1 receptor agonist and L-form of peneracin can effectively alleviate the cognitive dysfunction of SAMP8 mice. Natividad et al. Regular exercise is an alternative intervention measure to maintain the blood sugar level in the normal range and reduce the risk factors. Hsu and colleagues found that exercise combined with probiotics intervention can have a positive effect on blood sugar and increase insulin sensitivity in mice. The above results show that drug intervention, probiotic supplementation and intensive exercise can improve IR but more clinical data are still needed. Overall, the increased incidence of IR and the key roles of IR plays in many diseases, urgently require a better understanding of IR pathogenesis in addition to how IR interacts with genetics and different environments. A deeper understanding of IR can be achieved with a more systematic approach involving large-scale omics to study the molecular landscape is of major importance in addition to exploring new intervention strategies to prevent abnormal IR syndrome. Banting, F. The internal secretion of the pancreas. Indian J. CAS PubMed Google Scholar. SANGER, F. The amino-acid sequence in the glycyl chain of insulin. The identification of lower peptides from partial hydrolysates. Biochem J. Article CAS PubMed PubMed Central Google Scholar. The investigation of peptides from enzymic hydrolysates. Kung, Y. Total synthesis of crystalline bovine insulin. Goeddel, D. et al. Expression in Escherichia coli of chemically synthesized genes for human insulin. USA 76 , — Vecchio, I. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol. Article Google Scholar. Cheatham, B. Insulin action and the insulin signaling network. Root, H. Insulin resistance and bronze diabetes. Laakso, M. Insulin resistance and hyperglycaemia in cardiovascular disease development. Article CAS PubMed Google Scholar. Bugianesi, E. Insulin resistance in nonalcoholic fatty liver disease. Saklayen, M. The global epidemic of the metabolic syndrome. Diamanti-Kandarakis, E. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Stenvers, D. Circadian clocks and insulin resistance. Article PubMed CAS Google Scholar. Freeman AM, Pennings N. Insulin Resistance. In: StatPearls Internet. Treasure Island FL : StatPearls Publishing. PMID: American Diabetes Association. Prevention or delay of type 2 diabetes: standards of medical care in diabetes Diabetes Care 44 , S34—S39 Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes Diabetes Care 44 , S—S Weiss, M. Insulin biosynthesis, secretion, structure, and structure-activity relationships. In: Feingold KR, Anawalt B, Boyce A, et al. South Dartmouth MA : MDText. com, Inc. Sanger, F. Chemistry of insulin. Science , — Katsoyannis, P. Synthesis of insulin. Lee, J. The insulin receptor: structure, function, and signaling. Pessin, J. Signaling pathways in insulin action: molecular targets of insulin resistance. Invest , — Haeusler, R. Biochemical and cellular properties of insulin receptor signalling. Cell Biol. White, M. Mechanisms of insulin action. In Atlas of diabetes pp. Springer, Boston, MA Newgard, C. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase Diabetes 49 , — Beurel, E. Glycogen synthase kinase-3 GSK3 : regulation, actions, and diseases. Article CAS Google Scholar. Dong, X. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. Puigserver, P. Insulin-regulated hepatic gluconeogenesis through FOXO1—PGC-1α interaction. Nature , — Vander Haar, E. Garami, A. Cell 11 , — Laplante, M. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. USA , — Han, Y. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Calejman, C. mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis. Xia, W. Loss of ABHD15 impairs the anti-lipolytic action of insulin by altering PDE3B stability and contributes to insulin resistance. Cell Rep. James, D. The aetiology and molecular landscape of insulin resistance. Tam, C. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes care 35 , — Samuel, V. Mechanisms for insulin resistance: common threads and missing links. Cell , — Ye, J. Mechanisms of insulin resistance in obesity. Front Med. Article PubMed PubMed Central Google Scholar. Yaribeygi, H. Insulin resistance: Review of the underlying molecular mechanisms. Cell Physiol. De Meyts, P. The insulin receptor: a prototype for dimeric, allosteric membrane receptors? Trends Biochem Sci. Caro, J. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. Invest 79 , — Fröjdö, S. Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochim Biophys. Acta , 83—92 Fisher, S. Michael, M. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Cell 6 , 87—97 Davis, R. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser Article PubMed Google Scholar. Carvalho-Filho, M. Diabetes 54 , — Taniguchi, C. Critical nodes in signalling pathways: insights into insulin action. Brachmann, S. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. CAS PubMed PubMed Central Google Scholar. Czech, M. Signaling mechanisms that regulate glucose transport. Luo, J. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cong, L. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Xia, J. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Le Marchand-Brustel, Y. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Brozinick, J. Defective signaling through Akt-2 and-3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes 52 , — Kruszynska, Y. Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. Choi, K. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J. Intern Med. Kahn, B. Glucose transport: pivotal step in insulin action. Diabetes 45 , — Dimitriadis, G. Insulin effects in muscle and adipose tissue. Diabetes Res Clin. Shepherd, P. Glucose transporters and insulin action-implications for insulin resistance and diabetes mellitus. Li, J. Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice. FASEB J. Klip, A. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem Biophys. Res Commun. Etgen, G. Jr et al. Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to the cell surface. Ryder, J. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin-and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Garvey, W. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes: heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes 42 , — Chadt, A. Deletion of both Rab-GTPase—activating proteins TBC14KO and TBC1D4 in mice eliminates insulin-and AICAR-stimulated glucose transport. Diabetes 64 , — Chen, S. Tramunt, B. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63 , — Greenhill, C. Sex differences in insulin resistance. Qiu, J. Estradiol protects proopiomelanocortin neurons against insulin resistance. Endocrinology , — Zidon, T. Effects of ERβ and ERα on OVX-induced changes in adiposity and insulin resistance. Ikeda, K. Functions of estrogen and estrogen receptor signaling on skeletal muscle. Steroid Biochem Mol. Gerdts, E. Sex differences in cardiometabolic disorders. Chia, C. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Shi, H. Sex differences in obesity-related glucose intolerance and insulin resistance. Glucose Tolerance 4 , 37—66 Google Scholar. Geer, E. Gender differences in insulin resistance, body composition, and energy balance. Christen, T. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides , 25—31 Palmisano, B. Sex differences in lipid and lipoprotein metabolism. Kodama, K. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 36 , — Raygor, V. Diab Vasc. Sumner, A. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis , — Tan, V. Ethnic differences in insulin sensitivity and beta-cell function among Asian men. Diabetes 5 , e—e Ministry of Health Singapore MOHS. Potts, J. Sex and ethnic group differences in fat distribution in young United Kingdom South Asians and Europids. Ehtisham, S. Ethnic differences in insulin resistance and body composition in United Kingdom adolescents. Lear, S. Ethnic variation in fat and lean body mass and the association with insulin resistance. Mason, C. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Med 41 , — Mikusova, V. Insulin resistance and need for a lifestyle change to eliminate it. Listy , — orpeleijn, E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Shigeta, H. Lifestyle, obesity, and insulin resistance. Diabetes Care 24 , Oosterman, J. The circadian clock, shift work, and tissue-specific insulin resistance. Endocrinology , bqaa McAuley, K. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes care 25 , — Bergman, B. Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes 61 , — Kan, C. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes care 36 , — Sung, C. Role of vitamin D in insulin resistance. Ardabili, H. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Pasieka, A. Impact of glucocorticoid excess on glucose tolerance: clinical and preclinical evidence. Metabolites 6 , 24 Article PubMed Central CAS Google Scholar. Rizza, R. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. Effects of growth hormone on insulin action in man: mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 31 , — Barbour, L. A Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30 , S—S Parichatikanond, W. Prolonged stimulation of β2-adrenergic receptor with β2-agonists impairs insulin actions in H9c2 cells. Walli, R. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIVinfected patients. AIDS 12 , F—F Murata, H. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. Teff, K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes 62 , — Bittencourt, M. Insulin therapy in insulin resistance: could it be part of a lethal pathway? Elbein, S. Heritability of pancreatic beta-cell function among nondiabetic members of Caucasian familial type 2 diabetic kindreds. Shulman, G. Cellular mechanisms of insulin resistance. Knauf, C. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. Petersen, M. Regulation of hepatic glucose metabolism in health and disease. Matsumoto, M. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. Shimomura, I. Cell 6 , 77—86 Petersen, K. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. Henriksen, E. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Drug Targets 7 , — Karim, S. Hepatic expression and cellular distribution of the glucose transporter family. World J. Rencurel, F. Requirement of glucose metabolism for regulation of glucose transporter type 2 GLUT2 gene expression in liver. Thorens, B. Diabetologia 58 , — Eberlé, D. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86 , — Horton, J. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Ferré, P. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Tobe, K. Dentin, R. Carbohydrate responsive element binding protein ChREBP and sterol regulatory element binding protein-1c SREBP-1c : two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie 87 , 81—86 Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. Herman, M. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Iizuka, K. Deficiency of carbohydrate response element-binding protein ChREBP reduces lipogenesis as well as glycolysis. Natl Acad. Jaworski, K. Regulation of triglyceride metabolism. Hormonal regulation of lipolysis in adipose tissue. Liver Physiol. Vaughan, M. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. Zmuda-Trzebiatowska, E. Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal 18 , — Choi, Y. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B—null mice. Martinez-Botas, J. Genet 26 , — Tansey, J. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. USA 98 , — Mechanisms of Insulin Action and Insulin Resistance. Kimball, S. Regulation of protein synthesis by insulin. Pösö, A. Multifunctional control of amino acids of deprivation-induced proteolysis in liver. Role of leucine. Marshall, S. New insights into the metabolic regulation of insulin action and insulin resistance: role of glucose and amino acids. Rudrappa, S. Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance-a qualitative review. Front Physiol. Medeiros, C. |
Video
Insulin Signaling (Signal Pathways)
Nach meiner Meinung sind Sie nicht recht.
Mir scheint es die glänzende Phrase
Ich � dieser Meinung.