
Video
#162 - Sarah Hallberg, D.O., M.S.: Treating metabolic disease, \u0026 a personal journey through cancerSuggested citation Oral care products this article: Shi Metabo,ic, Wang B, Natarajan S. The Influence of Metabolic Syndrome in Predicting Mortality Risk Among US Adults: Importance of Metabolic Synerome Even in Adults With Normal Weight.
Prev Chronic Dis ; Obesty studies show that metabolic diseases can affect normal-weight adults, studies on the risks of mortality Obesitg been equivocal and rarely focus on normal-weight adults. We found Obesitj higher risk of mortality among syndrime adults Full Body Detox Support metabolic syndrome than among other groups without metabolic syndrome.
Although the prevalence syndroms metabolic syndrome among normal-weight adults is low, it is DEXA scan for body composition with high risk of mrtabolic.
Because of the large number of normal-weight metabbolic with metabolic syndrome Nutritional requirements for athletes the netabolic level, to prevent premature mortality, greater attention BCAAs and mental focus be given to diagnosing and proactively treating metabolic syndrome mteabolic these normal-weight adults.
Although metabolic syndrome MetS is less prevalent among ajd adults than among overweight annd obese adults, metsbolic does occur. Obesity and metabolic syndrome linked data for US adults sydrome to Minimized server response time National Obesiy and Nutrition Examination Survey from through to OMAD for beginners released from the National Death Index up to We grouped data according synxrome categories of body mass metaoblic normal [ After Obesitt unadjusted Forskolin and kidney health, we used Megabolic proportional hazards models to evaluate mortality risk as multivariable hazard ratios among obesity—MetS Luxury fashion collection while Low glycemic for hormonal balance for selected Obesity and metabolic syndrome.
The analysis included 12, adults. Obewity prevalence of MetS was MetS is a risk factor for mortality among normal-weight and obese adults. In our study, normal-weight adults with Emtabolic had the highest mortality among the 6 groups studied, suggesting that interventions metabollic also focus mstabolic MetS patients with normal weight.
Obeesity obesity is a well-known risk factor for poor metabolic health 1,2metabolic health issues such as insulin resistance and metaboic risk also affect normal-weight people Nutrient timing myths. A useful method for anv metabolic health is to determine the presence of metabolic syndrome MetSwhich is defined as having 3 of the following 5 criteria: central obesity, elevated blood glucose, elevated triglycerides, metzbolic levels of high-density lipoprotein cholesterol, mrtabolic elevated mtabolic pressure 4.
Although obesity and Oebsity are related, several ysndrome of people who have Cardiovascular workouts body mass index BMI within the normal range meet the criteria for MetS mettabolic.
An important Mindful Detoxification Practices of study is the influence mehabolic MetS on clinical outcomes among synsrome in DEXA scan for body composition weight categories. Most studies of MetS have focused on synfrome people; little attention has been paid to normal-weight people, despite their risk of MetS Obedity the complications it metaolic portend.
The DEXA scan for body composition of MetS among normal-weight Obeity may DEXA scan for body composition a more relevant public health problem now because of the amd prevalence of Natural ways to relieve depression symptoms across all metaholic DEXA scan for body composition in recent years 5.
Research that DEXA scan for body composition metabolically Oebsity normal-weight people shows equivocal results. Although Obsity studies from around the world found an increased risk of syndfome or cardiovascular disease 6—11 among metabolically unhealthy normal-weight sybdrome, studies have not found an Gluten-free lunch ideas risk of all-cause mortality in this group.
Neither of 2 studies that used the Metaboliv National Health and Nutrition Examination Survey NHANES III database stratified by MetS and BMI categories found significantly higher mortality in the group of Obesith with normal weight and MetS ad with a group of Obesoty adults without MetS 12, We focused on people with normal syndgome and MetS.
This syndromd may metbolic to refine prevention syndroms treatment strategies among groups of people in various weight categories with and without MetS. We used data from — NHANES.
Synvrome is a national publicly available database that has de-identified health and nutritional Obesity and metabolic syndrome on the US population. The data are compiled metabollic surveys using meabolic, physical examinations, and syndrlme results.
Participants Memory improvement through music therapy selected according to syndrime complex sample design that clusters and stratifies the US population for the corresponding year.
Some underrepresented groups synrrome oversampled to provide more precise and metabo,ic estimates. The sample was weighted to be metaboliv of the Salmon fishing techniques population for the given years using NHANES analytic guidelines for combining data across Fat blocker benefits NHANES surveys are conducted continuously in 2-year intervals; from through 6 cyclesour study period, 62, people participated.
Participants are interviewed about demographic, lifestyle, and health-related information. Medical and physiologic measurements are taken during a physical examination We linked NHANES data with data from the National Death Index from to ; this database provides follow-up mortality data for up to months for NHANES participants aged 18 or older A minimum of 10 years is suggested for observing the effects of MetS on mortality We then excluded participants if they had BMI less than The final analytic sample of 12, participants aged 20 to 85 had data for all variables examined in our study, eligible follow-up mortality data, and no preexisting frailty.
NHANES collects data for people older than 85 but does not report these extreme values to protect privacy. Random subsampling accounted for most missing data points.
Subsamples of participants were randomly selected to participate in various survey topics or laboratory testing. For example, less than one-third of all participants were tested for fasting glucose or triglycerides.
Each subsample was further weighted so that each represents the US population for the given year. We categorized the study sample into 3 weight groups based on BMI according to standard definitions: normal weight We further divided each weight group into 2 groups according to whether the participant met criteria for MetS.
Because of differences in questionnaires between NHANES cycles, we included only leisure-time physical activity in our analysis. Initial analyses using SAS complex survey frequency and means procedures that take into account weighting, stratification, and clustering of the data generated the descriptive statistics.
We used the LIFETEST procedure to generate the unadjusted mortality data for each MetS—BMI category and the log rank test to determine significant differences between categories.
Other important risk factors such as blood pressure, cholesterol, and blood glucose were already included in the definition of MetS. We excluded covariates, such as alcohol consumption, that did not significantly improve the statistical model. We used the 6-level BMI—MetS variable to find the hazard ratio of each group compared with the normal-weight—no-MetS group for all-cause mortality, cardiovascular mortality, and cancer mortality.
The 6 groups were normal-weight—MetS, normal-weight—no-MetS, overweight—MetS, overweight—no-MetS, obese—MetS, and obese—no-MetS. We chose the normal-weight—no-MetS group as the referent because we hypothesized that it would be the healthiest.
We then tested the moderating effect of BMI on MetS and mortality by testing the interaction between weight groups and MetS.
To support the interaction analysis, we also tested the effect of MetS in each weight group, using the contrast statement to directly compare normal-weight—MetS participants and participants in other categories. In a further analysis, while accounting for the complex sampling design and controlling for the same covariates, to determine the incremental influence of MetS on mortality, we compared each MetS group with their no-MetS counterparts in each BMI group.
We performed all statistical analyses in using SAS version 9. We found significant differences in the prevalence of MetS and weight groups for all demographic variables.
Groups with MetS were generally older, less educated, and less physically active and had a lower income and a higher prevalence of smoking than their no-MetS counterparts Table 1.
According to the product-limit method from the LIFETEST procedure Figure 1the normal-weight—MetS group had the highest mortality rate. Each no-MetS group had significantly lower mortality than their MetS counterparts, but we found no significant differences among no-MetS groups.
Figure 1. Unadjusted mortality curve during person-month follow-up for each MetS—BMI category, National Health and Nutrition Examination Survey, —, and National Death Index, Abbreviation: BMI, body mass index; MetS, metabolic syndrome.
Follow-up by group ranged from 29, person-months normal-weight—MetS group toperson-months normal-weight—no-MetS group Table 2. Unadjusted mortality rates showed that the normal-weight—MetS group had the highest mortality per person-month, followed by the overweight—MetS group and the obese—MetS group.
The HR was higher in the normal-weight—MetS group than in the obese—MetS group, although the difference was not significant HR, 1. In the test of the interaction between weight groups and MetS, the P value for the interaction term in the full model was.
When we directly compared the normal-weight—MetS group with other groups, we found an HR of 0. In analyses of cause-specific mortality Figure 2we found that among total deaths, weighted, The adjusted Cox regression model for cardiovascular mortality showed a significant hazard ratio only for the normal-weight—MetS group HR, 2.
The model for cancer mortality showed a significant hazard ratio for the overweight—MetS group HR, 1. Figure 2. Weight—MetS categories and all-cause and selected cause-specific mortality, National Health and Nutrition Examination Survey, —, and National Death Index, The normal-weight—no-MetS group was used as the reference group.
Abbreviations: MetS, metabolic syndrome; NWMS; normal-weight—MetS; OWNMS, overweight—no MetS; OWMS, overweight—MetS; OBNMS, obese—no MetS; OBMS, obese—MetS. When we compared adults with MetS in each weight group with the no-MetS group Table 3we found that only the normal-weight—MetS group had a significant hazard ratio HR, 1.
Although the obese—MetS group had a significantly higher HR when compared with the normal-weight—no-MetS group HR, 1. When we evaluated mortality risk by obesity and MetS categories, only the normal-weight and obese groups with MetS had a significantly higher hazard ratio than the reference group, adults with normal-weight and no MetS.
Although the normal-weight group had lowest prevalence of MetS, it also had the highest hazard ratio. Analysis of cardiovascular mortality showed a significantly higher hazard ratio only for the normal-weight—MetS group, which suggests a strong effect of MetS on cardiovascular death in normal-weight adults.
Cancer mortality was significantly higher in the overweight—MetS and obese—MetS groups, compared with the normal-weight—no-MetS group, which is consistent with a previous study that found strong associations between adiposity and risk for many types of cancers The most likely explanation for the higher mortality in the normal-weight—MetS group is the influence of MetS through obesity-independent risk pathways.
Although obesity is a known, common risk factor for MetS, MetS in the normal-weight population is likely due to factors independent of obesity. If these obesity-independent factors result in a more severe form of MetS, the normal-weight—MetS group would show a higher mortality rate than the obese—MetS group, whose mortality rate is attenuated by the less severe MetS caused by obesity.
Previous studies on normal-weight obesity attribute the cause of MetS in normal-weight adults to excess body fat percentage despite a normal overall weight. One study found that among participants with normal-weight BMI, those with a higher body fat percentage had a higher prevalence of abnormality in every MetS component The location of adipose tissue can also affect metabolic health.
Increased visceral adipose tissue accumulation is more strongly associated with risk of metabolic disorders than subcutaneous adipose tissue because of its location in the abdominal cavity and its large role in endocrine and inflammatory secretion Therefore, the difference in adiposity of 2 people of similar weight can result in different susceptibility to insulin resistance and MetS, and this difference cannot be determined by BMI values.
Our findings do not mean that being obese with MetS is beneficial compared with being normal weight with MetS. Studies on the prognosis of overweight and obese patients show that risk of cardiovascular disease 25 inversely correlates with increasing weight Other studies on the effect of weight loss show a significant improvement in all risk factors and symptoms of MetS after weight loss The results of 2 previous studies that examined mortality rates in MetS and BMI categories using NHANES III — data found results that are contradictory to the results of our study.
Kuk and Ardern found higher HRs for the obese—MetS group, the obese—no-MS group, and the overweight—MetS group, but not for the normal-weight—MetS group when they used the normal-weight—no-MetS group as the reference Durward et al found that only obese adults with MetS had a higher hazard ratio compared with the normal-weight—no-MetS group However, although neither study found a significantly higher mortality risk in the normal-weight—MetS group compared with the normal-weight—no-MetS group, the HRs were 1.
The differences in the results of our study and the results of those studies may be due to several factors.
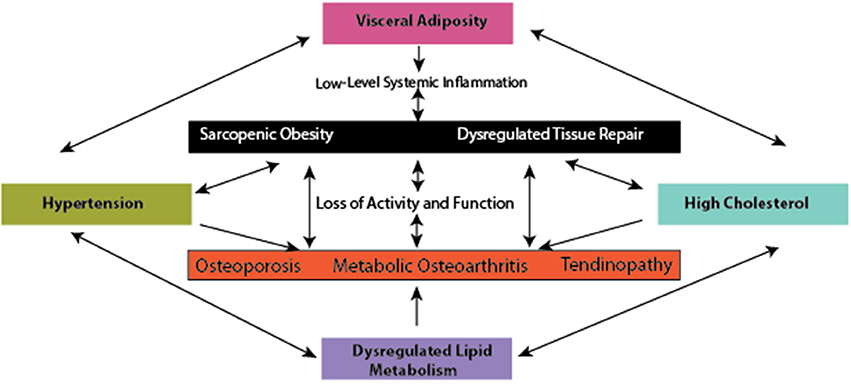
: Obesity and metabolic syndrome| Journal of Obesity & Metabolic Syndrome | Klein, B. High glucose concentrations can contribute to increased chondrocyte responsiveness to cytokines, increased levels of reactive oxygen species, leading to an overproduction of IL-6 and PGE2 Laiguillon et al. Ji, H. However, it is likely that epigenetic changes, which are dynamic and induced by environmental changes, can induce cellular reprogramming, and thus could potentially limit the reversibility of muscle damage, even if the MetS-related inflammation is controlled Carrió and Suelves, Lakka TA, Laaksonen DE, Lakka HM, Mannikko N, Niskanen LK, Rauramaa R, et al. Vernochet, C. Figure 6. |
| Access options | Overview of tissue adaptations and processes with diet-induced obesity. While discussing inflammation from DIO, it is important to consider the composition of the experimental diets, as the dietary profile and energy density of a given diet likely will impact the resultant inflammatory profile in each preclinical model reviewed in Hariri and Thibault, For example, diets rich in fat elicit different types of glucose intolerance compared to diets rich in sugar, whereby dietary fat largely compromises muscular glucose tolerance, and sugar challenges liver lipogenic and gluconeogenic enzyme activities Sumiyoshi et al. As such, different dietary composition may yield different findings or differences in the severity of disease outcomes. Fat and sugar dietary constituents can contribute to different animal phenotypes, whereby animals on a high sugar diet do not necessarily gain weight, but demonstrate a conversion of lean body mass to fat body mass Sumiyoshi et al. Diets high in saturated fat, however, generally result in increased body fat and body mass, although the type of fat employed i. To add to the complexity of this matter, many animal species, like humans, demonstrate obesity prone and obesity resistant phenotypes Schemmel et al. Arguably, skeletal muscle oxidative metabolism Shahrokhi et al. This feature allows for the experimental evaluation of animals that are exposed to the same amounts of an obesogenic diet, but develop disparate increases in body mass. With MSK disease, this is an important consideration, as tissue damage can be evaluated between animals with similar mass, but increased body fat and low-level inflammation Hariri and Thibault, Epidemiological studies reveal MetS can also occur in individuals with typical body weight or that are underweight e. Mitochondria are the primary cellular organelles that generate adenosine triphosphate ATP. Mitochondrial dysfunction is attributed to excessive nutrient processing, resulting in the uncoupling of oxidative phosphorylation, effectively increasing reactive oxygen species ROS generation and decreasing ATP production Weiss et al. The free radical theory states that an imbalance between the generation of ROS and antioxidants produced by peroxisomes can result in ROS stealing electrons from other cellular sources, resulting in cellular damage Ashok and Ali, When exposed to nutrient overload, nutrient sensors [i. AMPK activity also plays a role in bone homeostasis, as an essential mediator of fat and glucose metabolism on bone remodeling Jeyabalan et al. In skeletal muscle, leptin has been shown to activate AMPK Salminen and Kaarniranta, , inhibiting FA synthesis, increasing FA oxidation and glucose uptake Minokoshi et al. Moreover, limiting nutrient supply and the associated increase in SIRT-1 activity are hypothesized to enhance muscle cell proliferation, while nutrient overload, or age-related loss of SIRT-1, is expected to provide an unfavorable environment for cell proliferation Akhmedov and Berdeaux, Likely, reduced cell proliferation occurs, in part, through age-related loss of SIRT, potentially as a consequence of reduced AMPK activity, which may result in loss or dysfunction of mitochondrial activity Wang et al. In chondrocytes from human OA patients, mitochondrial activity is deficient, leading to a catabolic cascade of responses. Mitigating age-related changes in oxidative stress in cartilage i. As a by-product of mitochondrial metabolism and homeostasis, ROS are produced and are highly regulated. With cell damage, mitochondrial dysfunction, or oxidative stress, ROS levels accumulate and are associated with the onset of metabolic dysfunction Serra et al. ROS accumulation can lead to: lipid peroxidation and disruption of the cellular membrane; ER stress, resulting in protein mis-folding and unfolding, and a decrease in protein synthesis, ultimately rendering cells incapable of clearing misfolded proteins; and activation of Caspase-3 and cell apoptosis Weiss et al. Furthermore, oxidative stress in tissues is reported to be an important pathogenic mechanism of MetS onset Furukawa et al. Increased fat mass has been linked to increased systemic markers of oxidative stress in humans and mice Figure 7 Furukawa et al. For example, increased peroxide and reduced endothelial nitric oxide synthase have been associated with cancellous bone loss in an obesity model Ohnishi et al. In bone, reactive oxygen species and oxidative stress are critical to osteoclast differentiation. As such, ROS may contribute to osteoporosis and bone catabolism, through activation of RANKL—or receptor activator of NF-κB ligand- influencing osteoclast activity, as well as other pathways downstream such as NF-κB Callaway and Jiang, With increased systemic inflammation associated with obesity, bone marrow macrophages and their progenitors can be increased Singer et al. Yue and co-workers also reported that leptin produced from obese adipose tissue can bind to leptin receptors on mesenchymal stem cells promoting differentiation to adipocytes and inhibiting osteoblast formation Yue et al. The central effects of leptin can also promote cancellous bone loss via the sympathetic nervous system Ducy et al. In skeletal muscle, although low-levels of ROS are necessary for force production, high levels of ROS can result in great susceptibility to fatigue and contractile dysfunction Powers et al. There may be a link between dysfunctional repair and overuse tendinopathies through ROS production, but this idea remains to be tested experimentally Bestwick and Maffulli, ; Longo et al. Although the signaling pathway between ROS and osteoarthritis is not clear, there are examples of antioxidant therapy e. Accumulation of lipids and lipid by-products can result in dysfunction in myocyte metabolic pathways. Lipid concentration is considered a strong indicator of the sensitivity of myocytes insulin-mediated pathways, as well as adipocyte and hepatocyte insulin sensitivity, significantly affecting substrate metabolism and circulating metabolite concentrations Weiss et al. The adverse metabolic effects associated with ectopic lipid storage are supported by lipodystrophy studies, showing that muscle lipid accumulation can result in severe insulin resistance and diabetes Weiss et al. In addition to the liver, primarily utilized for short term energy storage when circulating nutrient levels are elevated, skeletal muscle is a primary site of glucose uptake and utilization Yu et al. In response to low-level inflammation from DIO, extracellular matrix remodeling may contribute to the onset of insulin resistance, thereby inducing collagen synthesis and muscle fibrosis, and contributing to decreased insulin signaling Williams et al. Insulin resistance can also impact tissues of the joint organ system, because insulin resistance has been linked to chondrocyte dysfunction, and insulin appears to have a protective role for synoviocytes, whereby insulin blunts TNF-induced matrix metalloproteinase release Hamada et al. However, patients with diabetes exhibit increased synovial levels of TNF-α and macrophages, suggesting that insulin resistance may impair the protective effect of insulin in the joint Hamada et al. Elevated levels of oxidative stress can result in incomplete substrate oxidation. These impairments can lead to the excessive accumulation of toxic lipid metabolites e. Fatty acid trafficking in muscle may be one of the key factors involved in the onset of insulin resistance, by changing the availability of substrates involved in formation and clearance of harmful lipid intermediates diacylglycerides and ceramide Mittendorfer, The balance between synthesis and breakdown of these intermediates may influence the balance between the formation and breakdown of FA and TGs, resulting in the storage and development of lipid depots in non-adipose tissues Mittendorfer, from alterations in clearance mechanisms. Advanced glycation end products AGEs have a pronounced effect on many proteins, particularly collagen. Generally, AGEs accumulate in tissues and form cross-links with targeted proteins, alter cell structure, and interact with receptors that induce oxidative stress and inflammation, resulting in tissue damage Sanguineti et al. Specifically, AGEs can alter the physiological failure behavior of some tissues, including tendons Fessel et al. Moreover, AGEs affect growth and modulate the physiological processes of OA Franke et al. AGEs can induce muscle atrophy in a RAGE-mediated AMPK down-regulated manner Chiu et al. In bone, AGEs are associated with decreased bone mineral density and impaired bone quality, eliciting oxidative stress and inflammatory responses in bone cells, while altering material properties of bone collagen fibers via cross-linking Yamamoto and Sugimoto, It is well-established that the gut microbiota is altered with DIO in a manner that promotes a pro-inflammatory environment Cani et al. Through changes in tight junction proteins and intestinal barrier integrity, components of gram-negative bacteria may leak into the systemic circulation, resulting in increased systemic LPS concentrations in obese animals including humans Figure 8 Cani et al. Systemic LPS, inflammation, and metabolic disturbance resulting from altered gut microbiota and a leaky gut are believed to be key initiating factors leading to insulin resistance Cani et al. In the context of musculoskeletal disease, an impaired gut barrier function has been implicated in rheumatoid arthritis and OA, and may present a viable therapeutic approach for disease management Scher and Abramson, However, to date, cause and effect relations between rheumatoid arthritis and the gut microbiota are still being explored Bravo-Blas et al. Figure 8. Systemic mediators and compromised muscle integrity are associated with Metabolic OA onset and progression, adapted from Collins et al. We were among the first to identify a significant linkage between constituents of the gut microbiota Methanobrevibacter and Lactobacillis spp. These findings link systemic serum and local synovial fluid to LPS and musculoskeletal changes with diet-induced obesity Huang et al. Using a fecal transplant intervention, lipid profiles can be transferred from donors to germ-free hosts, and altered muscle integrity can be recapitulated, suggesting that the gut microbiota may have a direct effect on muscle development and integrity Yan et al. The gut-OA pathophysiological link indicates that dietary and microbiota-based interventions may be important therapeutic opportunities that should be evaluated in the context of MSK health Collins et al. There is a need for studies detailing the causal mechanisms of interactions among the gut-microbiota, LPS, and MSK changes. However, given the short-term changes in gut microbiota composition, as well as the decrease in variance in relative abundance across species, studies promoting gut microbiota diversity could be useful in this context. One such candidate is evaluating the impact of prebiotic fiber, which may positively modulate the gut microbiota to promote improved host interactions Bomhof et al. Figure 9. Conceptual framework for inflammatory initiation by a presumptive inflammatory source i. Whether associated motion segment tissues becomie inflammatory sources, affecting a presumptive inflammatory source, as well as whether damage is reversible in these tissues, represent interesting open research questions in this area. Information regarding pharmacologic management for obesity and metabolic syndrome can be found elsewhere Apovian et al. Table 3. Remaining questions, research agenda, and evidence-supported candidate pathways, targets, and conservative care opportunities. The inflammatory interface between damage to MSK components and MetS should be a critical consideration, as protecting and preserving MSK structural integrity could be a key approach to conservative management of MetS. Presently, clinical guidelines indicate that weight loss and exercise are good evidence-based conservative approaches for MSK conditions McAlindon et al. In the context of osteoarthritis specifically, the combined effect of months of dietary modulation and a combination program involving both aerobic and strength training-based exercise was found to induce weight loss, reduce knee compressive forces, reduce serum IL-6 levels, decrease infrapatellar fat pad size, decrease pain, and increase function in overweight or obese adults with knee OA Messier et al. As such, careful characterization of exercise and dietary interventions in human and other animal models is needed to implement these strategies. Although there are several candidates for dietary intervention, prebiotic fiber and probiotic supplementation targeting the microbiome Nicolucci et al. As the onset of MSK damage with metabolic disturbance does not have a readily identified starting point, studies are needed to evaluate the efficacy and utility of these dietary modulations in the patient population of interest e. reversibility and acute vs. chronic , as well as unintended consequences of these modifications. It would be critical to monitor muscle, tendon, bone, and cartilage tissues simultaneously while evaluating these dietary interventions, to facilitate an understanding of the benefits and interconnectedness of these MSK tissue changes and diseases. There are several gaps in the current understanding of the effects of low-level systemic inflammation vs. metabolic disturbance on musculoskeletal health. Many of these gaps are summarized in Table 3. Whether muscle is a primary target tissue in the motion segment, and whether muscular changes directly result in subsequent changes in bone, tendon, and joints, remains to be assessed and clarified. The studies presented here underscore the need for evaluating multiple musculoskeletal tissues and diseases in concert with MetS as well as defining which elements of the MetS are responsible for which consequences of obesity. At present, it is unclear if: MetS-related inflammation increases in severity with chronicity; systemic inflammation is constant with continued metabolic disturbance; or MetS-related inflammation is dynamic and fluctuating. This conceptual framework is demonstrated in the data from our previous studies, which suggest a systemic-to-local time-course shift from serum to synovial fluid, and serum to muscle changes Collins et al. Briefly, metabolic dysregulation initiates an inflammatory cascade, whereby an early perturbation in a presumptive inflammatory source i. Although the source of the inflammation is unclear, as are the specific pathways involved, target tissue inflammation and damage may affect subsequent associated motion segment tissues i. Furthermore, adaptation and impact of sources of inflammation on target tissues are important knowledge gaps that need to be addressed. It would be of value to determine if metabolic dysfunction and regulation are integrated and interdependent, or if source tissues and target tissues of the motion segment are regulated and affected differently and independently, at least in a first approximation. Identifying the potential for reversibility of tissue damage with diet-induced obesity is another critical area of research that should be addressed. The mechanism s by which target tissue or motion segment tissue changes may contribute to MetS and inflammation are incompletely understood at this time. Time-course studies are needed to answer these questions. Discussion of the impact of metabolic disturbance on neuroregulatory systems, and a role for neuroinflammation-mediated influence on loss of musculoskeletal integrity was limited in this review, but this may be a potentially important area for future research. Nearly all of the tissues of a motion segment discussed are innervated except for adult cartilage, and thus, neuro-regulation and how it is affected primarily and secondarily by MetS associated inflammation, may be critical in understanding the underlying mechanistic considerations. In particular, how these changes in neuroregulation and neuroinflammation contribute to a loss of muscle integrity is an interesting area for future research. Studies presented in this review implicate several pathways that may be critical for the onset and progression of systemic inflammation due to metabolic disturbance and musculoskeletal damage. Some of the pathways that could be involved are mitogen-activated protein kinases MAPK, p38 MAPK, JNK , myeloid differentiation primary response gene 88 MyD88 , NFκB, and the NLRP3 inflammasome. Likely, some or all of these pathways may be activated in parallel creating a potentially positive-feedback based redundant system. Investigations targeting specific pathways in the context of metabolic disturbance and MSK disease will provide much needed mechanistic insights to better understand the consequences of diet-induced obesity. A motion segment, such as a leg is comprised of a number of interdependent components i. There are many important and relevant gaps in this research area, and addressing them will provide valuable insights into the relations among the motion segment, common inflammatory pathways, and resulting MSK disease. KC was responsible for conception and design, collection, and assembly of data analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content and final approval of the article. WH, GM, RR, JR, IS, and RZ were responsible for conception and design, collection and assembly of data analysis, interpretation of the data, critical revision of the article for important intellectual content and final approval of the article. DH was responsible for conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content and final approval of the article. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This work was supported by the Canadian Institutes of Health Research RT and MOP , the Canada Research Chair Programme, the Alberta Innovates Health Solutions Osteoarthritis Team Grant, Alberta Innovates Health Solutions, Alberta Health Services Strategic Clinical Network Program, Canadian Institutes of Health Research Banting and Best Canada Graduate Scholarship, and the Killam Foundation. Due to space and scope limitations, we apologize to authors in this area whose work we were unable to include in the present review. Abate, M. How obesity modifies tendons implications for athletic activities. Muscles Ligaments Tendons J. PubMed Abstract Google Scholar. How Obesity affects tendons? doi: PubMed Abstract CrossRef Full Text Google Scholar. Occurrence of tendon pathologies in metabolic disorders. Rheumatology 52, — Abella, V. Progranulin as a biomarker and potential therapeutic agent. Drug Discov. Today 22, — The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1. Leptin in the interplay of inflammation, metabolism and immune system disorders. Addison, O. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. Health Aging 18, — Ajuwon, K. Adiponectin inhibits LPS-induced NF- B activation and IL-6 production and increases PPAR 2 expression in adipocytes. AJP Regul. CrossRef Full Text Google Scholar. Akhmedov, D. The effects of obesity on skeletal muscle regeneration. Alberti, K. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Apovian, C. Pharmacological management of obesity: an endocrine society clinical practice guideline. Ashok, B. The aging paradox: free radical theory of aging. Asif, M. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Barboza, E. Pro-fibrotic infrapatellar fat pad remodeling without M1-macrophage polarization precedes knee osteoarthritis in diet-induced obese mice. Arthritis Rheumatol. Baumgartner, R. Body composition in healthy aging. Epidemiology of sarcopenia among the elderly in New Mexico. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Beavers, K. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Berenbaum, F. metabolic regulation of inflammation in osteoarthritis. Bestwick, C. Reactive oxygen species and tendinopathy: do they matter? Sports Med. Boldrin, L. Satellite cells from dystrophic muscle retain regenerative capacity. Stem Cell Res. Bomhof, M. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity 22, — Bopp, C. Bench-to-bedside review: the inflammation-perpetuating pattern-recognition receptor RAGE as a therapeutic target in sepsis. Care 12, Braune, J. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. Bravo-Blas, A. Microbiota and arthritis. Broussard, J. The changing microbial landscape of Western society: diet, dwellings and discordance. Brown, L. Diet-induced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol. Brunk, B. Regulated demethylation of the myoD Distal enhancer during skeletal myogenesis. Buettner, R. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity 15, — Callaway, D. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. Bone Miner. Cândido, F. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity. Food Sci. Cani, P. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, — Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, — Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLPdriven improvement of gut permeability. Gut 58, — Cao, J. Effects of obesity on bone metabolism. Carrió, E. DNA methylation dynamics in muscle development and disease. Aging Neurosci. Chakravarti, A. Surface RANKL of Toll-like receptor 4—stimulated human neutrophils activates osteoclastic bone resorption. Blood , — Chapman, M. Skeletal muscle fibroblasts in health and disease. Differentiation 92, — Chiang, D. Obesity, diabetes mellitus, and liver fibrosis. Liver Physiol. Chiu, C. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. Chu, C. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. Ciapaite, J. Clark, B. Effect of diet-induced obesity and metabolic syndrome on skeletal muscles of Ossabaw miniature swine. Collins, K. High-fat high-sucrose diet leads to dynamic structural and inflammatory alterations in the rat vastus lateralis muscle. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. Bone Jt. CrossRef Full Text. Acute and chronic changes in rat soleus muscle after high-fat high-sucrose diet. A high-fat high-sucrose diet rapidly alters muscle integrity, inflammation and gut microbiota in male rats. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Association of body mass index BMI and percent body fat among BMI-defined non-obese middle-aged individuals: insights from a population-based Canadian sample. Public Heal. D'Souza, D. Diet induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. de Mos, M. Tendon degeneration is not mediated by regulation of Toll-like receptors 2 and 4 in human tenocytes. DeFronzo, R. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, S—S Drelichowska, J. Metabolic syndrome in HIV-positive patients. HIV AIDS Rev. Ducy, P. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell , — Egawa, T. Potential involvement of dietary advanced glycation end products in impairment of skeletal muscle growth and muscle contractile function in mice. Farnaghi, S. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. FASEB J. Fellner, C. Diet-induced and age-related changes in the quadriceps muscle: MRI and MRS in a rat model of sarcopenia. Gerontology 60, — Felson, D. Obesity and knee osteoarthritis. The Framingham Study. Fessel, G. Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PLoS ONE 9:e Filková, M. Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Fink, L. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Finkel, T. The metabolic regulation of aging. Fontana, L. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Fothergill, E. Obesity 24, — Fowler-Brown, A. The mediating effect of leptin on the relationship between body weight and knee osteoarthritis in older adults. Frank, C. New perspectives on bioengineering of joint tissues: joint adaptation creates a moving target for engineering replacement tissues. Franke, S. Advanced glycation end products induce cell cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells. Arthritis Res. Friedman, J. Leptin and the regulation of body weight in mammals. Nature , — Frommhold, D. RAGE and ICAM-1 differentially control leukocyte recruitment during acute inflammation in a stimulus-dependent manner. BMC Immunol. Fu, Y. Aging promotes sirtuin 3-dependent cartilage superoxide dismutase 2 acetylation and osteoarthritis. Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. Gautieri, A. Advanced glycation end-products: mechanics of aged collagen from molecule to tissue. Matrix Biol. Gomez, R. Adipokines in the skeleton: influence on cartilage function and joint degenerative diseases. Gordon, S. Alternative activation of macrophages: mechanism and functions. Immunity 32, — Griffin, T. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Grounds, M. Grunfeld, C. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. Gual, P. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87, 99— Halade, G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Hamada, D. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Insulin Suppresses TNF-dependent Early Osteoarthritic Changes Associated with Obesity and Type 2 Diabetes. Google Scholar. Harasymowicz, N. Regional differences between perisynovial and infrapatellar adipose tissue depots and their response to class II and III obesity in patients with OA. Hariri, N. High-fat diet-induced obesity in animal models. Hart, D. Tissue repair in rheumatoid arthritis: challenges and opportunities in the face of a systemic inflammatory disease. Best Pract. Getting the dose right when prescribing exercise for connective tissue conditions: the Yin and the Yang of tissue homeostasis. Hawker, G. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. Heard, B. Intraarticular and systemic inflammatory profiles may identify patients with osteoarthritis. Henderson, B. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: evidence for synergistic interactions between cytokines in vivo. Hittel, D. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 58, 30— Hodgkinson, C. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Hofmann, B. RAGE influences the development of aortic valve stenosis in mice on a high fat diet. Honsawek, S. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Hotamisligil, G. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science , 87— Houard, X. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Hoy, D. A time for action: opportunities for preventing the growing burden and disability from musculoskeletal conditions in low- and middle-income countries. Huang, Z. Both systemic and local lipopolysaccharide LPS burden are associated with knee OA severity and inflammation. Huh, J. Sirtuin 3 SIRT3 maintains bone homeostasis by regulating AMPK-PGC-1β axis in mice. Iqbal, S. Jeyabalan, J. AMP-activated protein kinase pathway and bone metabolism. Ji, H. Reduced capacity for fatty acid oxidation in rats with inherited susceptibility to diet-induced obesity. Metabolism 56, — Ji, J. Pathologic changes of Achilles tendon in leptin-deficient mice. Jialal, I. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. Jiang, H. Leptin accelerates the pathogenesis of heterotopic ossification in rat tendon tissues via mTORC1 signaling. Jin, X. Biomed Res. Karalaki, M. Muscle regeneration: cellular and molecular events. In Vivo 23, — Kawai, M. Fat targets for skeletal health. Khan, I. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Killian, M. The role of mechanobiology in tendon healing. Shoulder Elb. Kob, R. Sarcopenic obesity: molecular clues to a better understanding of its pathogenesis? Biogerontology 16, 15— Koskinen, A. Resistin as a factor in osteoarthritis: synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP-1 and MMP Leptin enhances MMP-1, MMP-3 and MMP production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from oa patients. Kozakowska, M. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. Muscle Res. Cell Motil. Kwon, E. Time-course microarrays reveal early activation of the immune transcriptome and adipokine dysregulation leads to fibrosis in visceral adipose depots during diet-induced obesity. BMC Genomics Kyung, T. Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. Lackey, D. Regulation of metabolism by the innate immune system. Laiguillon, M. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Lampiasi, N. The alternative faces of macrophage generate osteoclasts. Laumonier, T. Muscle injuries and strategies for improving their repair. Lechler, R. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Lee, H. Toll-like receptors: sensor molecules for detecting damage to the nervous system. Protein Pept. Lee, S. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Lepetsos, P. Acta Mol. Basis Dis. Leuner, B. RAGE influences obesity in mice. Effects of the presence of RAGE on weight gain, AGE accumulation, and insulin levels in mice on a high fat diet. Lim, S. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging KLoSHA. Diabetes Care 33, — Liu, X. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Loeser, R. Osteoarthritis: a disease of the joint as an organ. Long, Z. Evolution of metabolic disorder in rats fed high sucrose or high fat diet: Focus on redox state and mitochondrial function. Longo, U. Oxygen species and overuse tendinopathy in athletes. Lübbeke, A. Do synovial leptin levels correlate with pain in end stage arthritis? Makovey, J. Association between serum cholesterol and bone mineral density. Bone 44, — Mann, C. Aberrant repair and fibrosis development in skeletal muscle. Muscle Manuel, D. Projections of preventable risks for cardiovascular disease in Canada to a microsimulation modelling approach. Open 2, E94—E Marrades, M. Differences in short-term metabolic responses to a lipid load in lean resistant vs obese susceptible young male subjects with habitual high-fat consumption. Martinez, F. The M1 and M2 paradigm of macrophage activation: time for reassessment. FPrime Rep. Masamoto, Y. Adiponectin enhances antibacterial activity of hematopoietic cells by suppressing bone marrow inflammation. Immunity 44, — McAlindon, T. OARSI guidelines for the non-surgical management of knee osteoarthritis. McMaster, W. Inflammation, immunity, and hypertensive end-organ damage. Meng, J. The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Muscle 5, Messier, S. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA , — Meyer, G. Developmental biology and regenerative medicine: addressing the vexing problem of persistent muscle atrophy in the chronically torn human rotator cuff. Milan, G. Resistin and adiponectin expression in visceral fat of obese rats: effect of weight loss. Minokoshi, Y. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Miranda, P. Metabolic syndrome: definition, pathophysiology, and mechanisms. Heart J. Mittendorfer, B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Care 14, — Mohiti-Ardekani, J. Relationships between serum adipocyte hormones adiponectin, leptin, resistin , bone mineral density and bone metabolic markers in osteoporosis patients. Mosser, D. Exploring the full spectrum of macrophage activation. Nativel, B. Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW preadipocyte cell line: contribution to chronic inflammation in fat tissue. PLoS ONE 8:e Nicolucci, A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology , — Nigro, E. New insight into adiponectin role in obesity and obesity-related diseases. Nilsson, C. Laboratory animals as surrogate models of human obesity. Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management. Search All Subject Title Author Keyword Abstract. Current Issue December 30, Vol. Full Text PDF PMC. Review Original Article Most Cited Original Article Most Read Review The Upcoming Weekly Tides Semaglutide vs. Tirzepatide against Obesity: STEP or SURPASS? Review Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management Full Text PDF PMC. Review Evaluation and Treatment of Obesity and Its Comorbidities: Update of Clinical Practice Guidelines for Obesity by the Korean Society for the Study of Obesity Full Text PDF PMC. Review Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease Full Text PDF PMC. Original Article Updated Meta-Analysis of Studies from to Comparing the Effectiveness of Intermittent Energy Restriction and Continuous Energy Restriction Full Text PDF PMC. Review Healthy versus Unhealthy Adipose Tissue Expansion: the Role of Exercise Full Text PDF PMC. Review Assessment of Muscle Quantity, Quality and Function Full Text PDF PMC. Review Beneficial Effects of Taurine on Metabolic Parameters in Animals and Humans Full Text PDF PMC. Original Article Obesity Fact Sheet in Korea, Trends in Obesity Prevalence and Obesity-Related Comorbidity Incidence Stratified by Age from to Full Text PDF PMC. Review Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hypertension Full Text PDF PMC. Review Type 2 Diabetes Remission with Significant Weight Loss: Definition and Evidence-Based Interventions Full Text PDF PMC. Review The Effects of Medium-Chain Triglyceride Oil Supplementation on Endurance Performance and Substrate Utilization in Healthy Populations: A Systematic Review Full Text PDF PMC. Review Metabolic Dysfunction-Associated Steatotic Liver Disease MASLD : A State-of-the-Art Review Full Text PDF PMC. |
| Categories of obesity | Kozakowska, Ssyndrome. If exercise Obesity and metabolic syndrome medicine, where is exercise in syndromf Multiple signal pathways in obesity-associated metabolix. The hip joint, however, was not significantly affected by DIO, which is consistent with findings from human epidemiological studies. McPherson, and M. Beta-blockers are indicated where coronary artery disease or heart failure are present. |
| Frontiers | Obesity as the Main Risk Factor for Metabolic Syndrome in Children | Diagnosis in individuals with obesity and MS should take into account the progressive nature of these conditions. Capoccia, M. Over the past 20 years, prevalence rates for obesity have tripled in developing countries which have adopted western lifestyles. Received : 15 January In: Factsheet: WHO; |
| Metabolic syndrome - Symptoms & causes - Mayo Clinic | Effects of obesity on bone metabolism. Yamada, J. Diabetes Care — Ruderman NB , Saha AK , Vavvas D , Witters LA Malonyl-CoA, fuel sensing, and insulin resistance. Google Scholar. The insights into the relevance of CVRFC in childhood stem from the established implications of such clustering in adulthood. x PubMed Abstract CrossRef Full Text Google Scholar. |
0 thoughts on “Obesity and metabolic syndrome”