
Video
Management of Hyperglycemic Crises in Adults with DiabetesHyperglycemic crisis and metabolic acidosis -
On one end of the spectrum lie absolute insulin deficiency and profound ketosis and acidosis, which is DKA. DKA tends to occur in patients with type 1 diabetes, who, because of destruction of β-cells, exhibit absolute insulin deficiency. On the other end of the spectrum is extreme hyperglycemia without ketosis and acidosis.
As the analogy implies, patients may present with various manifestations of both disorders. For example, a patient with DKA may have used enough insulin to partially suppress ketosis but still manifest profound hyperglycemia. Patients with HHS may also have varying degrees of ketosis and mild acidosis, depending on the degree to which they have been able to produce insulin and the extent of associated factors such as dehydration.
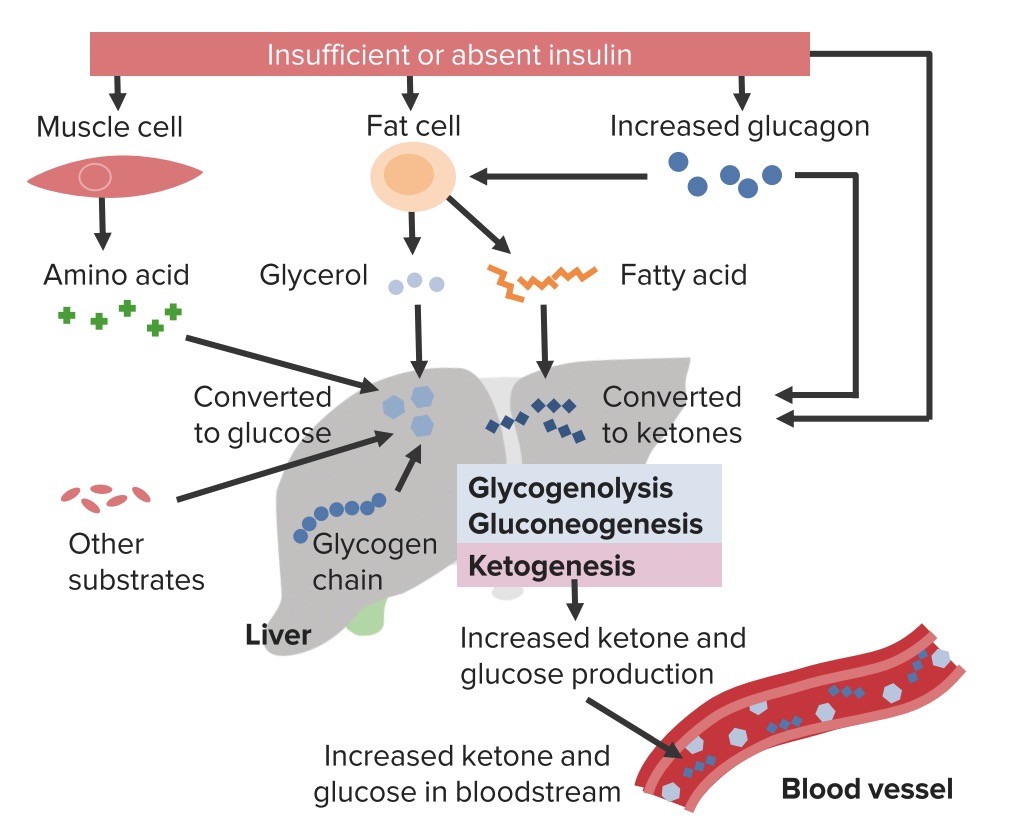
Insulin deficiency causes a lack of glucose utilization in insulin-dependent tissues such as muscle and adipose and therefore leads to hyperglycemia. Lack of insulin also stimulates hyperglycemia by increasing hepatic gluconeogenesis.
This is a common mechanism in both DKA and HHS. Deprived of glucose utilization, the body must look elsewhere for fuel to survive. In addition to hyperglycemia, lack of insulin increases degradation of triglycerides into free fatty acids in adipose tissue, which travel to the liver and are converted to the ketoacids β-hydroxybutyric acid, acetone, and acetoacetate.
Unopposed conterregulatory hormone effect causes further increases in glucose production from the liver and degradation of triglycerides. The surge of ketoacid formation from unrestrained ketone body formation can be profound. DKA develops when the surge of ketoacid production is so powerful that a metabolic acidosis results.
In HHS, there remains sufficient insulin presence to suppress ketosis enough to prevent the development of metabolic acidosis. Dehydration is another common finding in DKA and HHS. Because of osmotic pressure, unregulated diuresis follows.
Patients frequently complain of preceding polyuria and polydipsia. Considerable electrolyte loss may result, especially potassium depletion. Further dehydration and volume contraction can lead to worsening of hyperglycemia.
Patients presenting in HHS and DKA typically exhibit a history of polyuria and polydipsia. Frequently, one can identify a precipitating factor leading to DKA.
Such factors can include inappropriate use of insulin non-compliance , cardiovascular disease, or infection, which may be the most common causes of DKA. Patients with DKA may also manifest leukocytosis simply due to DKA.
It is important to not overlook other possible causes of DKA and HHS, however. Myocardial infarction may precipitate hyperglycemia and DKA via an increase in counterregulatory hormones, such as epinephrine. Drugs such as thiazides, sympathomimetics, second-generation antipsychotics, and corticosteroids may also precipitate HHS and DKA.
Other disorders that may precipitate diabetes include pancreatitis and illicit drug use. Additionally, and especially in patients with type 1 diabetes, decline in diabetes control and hyperglycemia may indicate the onset of an autoimmune thyroid disease, such as Grave's disease or Hashitoxicosis.
Patients may develop progressive hyperglycemia over weeks or days, although patients with DKA may experience more rapid onset than those with HHS. Symptoms of both HHS and DKA include polyuria and polydipsia due to hyperglycemia and signs of dehydration, including lack of skin turgor, hypotension, dry oral mucosae, tachycardia, weakness, and altered sensorium.
Patients with DKA typically exhibit signs of acidosis, such as abdominal pain sometimes severe , nausea, vomiting, and Kussmaul respirations, and may also exhibit guaiac-positive vomitus. Hypothermia, should it be present, is a poor prognostic indicator. Laboratory findings in patients with DKA include hyperglycemia, ketosis, and metabolic acidosis.
Patients who are suspected of DKA or HHS should undergo measurement of electrolytes with anion gap, glucose serological , creatinine and blood urea nitrogen, serum ketones, urinalysis with ketones, complete blood count, A1C, and arterial blood gas testing.
Additionally, electrocardiogram, chest X-ray, and urine, sputum, and blood cultures may be warranted. If children are otherwise healthy and there are no signs of infection, it may be acceptable to omit an infection workup. Significant ketosis has been shown in up to one-third of patients with HHS, again indicative of the continuum of pathology between DKA and HHS.
Buildup of ketoacids is responsible for anion gap metabolic acidosis in DKA. It is important, however, to remember other causes of anion gap metabolic acidoses, including starvation, lactic acidosis especially in patients using metformin , salicylates, ethanol, methanol, ethylene glycol, paraldehyde, renal insufficiency, and isopropyl alcohol intoxication.
Serum potassium levels are typically elevated in response to the presence of acidosis and insulin deficiency, but total body potassium is depleted. Patients presenting with hypokalemia in the setting of DKA are particularly potassium-depleted and require aggressive monitoring and potassium repletion.
Both amylase and lipase may be elevated in the setting of DKA and are not necessarily indicative of pancreatitis. The cornerstones of treatment of DKA and HHS are fluids, insulin, correction of electrolyte abnormalities, and close monitoring.
In the absence of underlying renal and cardiac disease, initial fluid resuscitation should consist of isotonic fluids to restore renal perfusion. Subsequently, fluids may be altered or titrated based on degree of dehydration and electrolyte abnormalities.
Titration of fluids is based on hemodynamic improvement, urine output, laboratory improvement, and clinical response. Patients with underlying cardiac and renal disease may require lower initial fluid resuscitation rates and more frequent monitoring of clinical status to avoid fluid overload.
Insulin therapy for DKA and HHS is typically administered intravenously, although uncomplicated mild to moderate DKA may be managed with subcutaneous insulin therapy.
Typically, in the absence of hypokalemia, patients receive a bolus of intravenous regular insulin at 0. Doses may be titrated based on clinical response, which will vary based on the degree of insulin resistance. For example, patients with type 2 diabetes who present in DKA will typically require a higher dose of insulin than those with type 1 diabetes because of higher insulin resistance.
If subcutaneous insulin is to be used to treat uncomplicated DKA, patients typically receive an initial dose of 0. Such approaches may be associated with a lower cost of hospitalization by avoiding intensive care unit placement. Electrolytes, glucose, blood urea nitrogen, osmolality, creatinine, and pH arterial or venous should be drawn every hours to monitor patients' responses to therapy and to allow titration of insulin and fluids.
It is important to note that hyperglycemia typically resolves before ketosis; therefore, dextrose should be added to fluids as glucose declines as described above. Ketosis should be measured via β-hydroxybutyric acid whenever possible because that is the prevalent ketone body produced in DKA.
The nitroprusside reaction, which is still used in many laboratories to detect ketone formation, does not detect β-hyrdoxybutyric acid and therefore may yield false-negative results.
Most patients presenting in DKA exhibit hyperkalemia as a result of insulin deficiency and acidosis despite total body potassium depletion. Treatment with insulin, restoration of normal circulatory volume, and resolution of acidosis allow total body potassium depletion to manifest itself as hypokalemia during treatment of DKA.
Including mEq of potassium in each liter of fluid is usually sufficient to maintain a potassium concentration within normal limits. This will help to avoid cardiac arrhythmia and skeletal muscle dysfunction because insulin initiation can cause an acute decline in serum potassium concentration.
Use of bicarbonate to raise pH is controversial. Patients also frequently exhibit hypophosphatemia at presentation in DKA, but phosphate repletion has not demonstrated a beneficial effect on clinical outcomes in DKA. Patients receiving phosphate therapy should be monitored closely for hypocalcemia, which can result from phosphate administration.
Patients should resume rapid-acting insulin at meals and intermediate- or long-acting insulin when they are able to eat substantial carbohydrate. It is important to continue intravenous insulin for several hours after resumption of subcutaneous insulin to avoid recurrent hyperglycemia and a possible return to ketosis.
The most common complications that occur when treating adults with ketoacidosis are hypokalemia and hypoglycemia.
Potassium depletion is the most life-threatening electrolyte abnormality in the treatment of DKA. As previously described, total body potassium at presentation in DKA is low despite hyperkalemia because of metabolic acidosis. Delayed potassium supplementation can lead to considerable hypokalemia as the serum potassium concentration drops precipitously in the presence of insulin and resolution of ketoacidosis.
In the setting of normal renal function, patients should receive potassium supplementation in their fluids when the potassium level approaches normal values. Hypoglycemia is also a potential complication of DKA. The threat of hypokalemia and hypoglycemia both also illustrate the importance of frequent reassessment of patients treated for DKA.
Care must also be taken in intravenous fluid administration. Patients with underlying medical conditions such as renal insufficiency or congestive heart failure are susceptible to fluid overload. Patients should be assessed for such disorders before initiation of fluid resuscitation.
Cerebral edema is yet another potential complication of DKA and HHS. It occurs more frequently in pediatric patients than in adults. Signs of cerebral edema include mental status changes, vomiting, headache, lethargy, elevated diastolic blood pressure, decorticate or decerebrate posturing, cranial nerve palsies, or Cheyne-Stokes respiration.
Treatment options include use of mannitol or hypertonic saline to decrease cerebral edema, although there have been no large controlled trials clearly demonstrating benefit. Hyperchloremic nonanion gap metabolic acidosis is a very frequent finding after resolution of DKA.
Diabetic ketoacidosis in type 2 diabetes mellitus — pathophysiology and clinical presentation. Nat Clin Pract Endocrinol Metab. Deeb A, Yousef H, Abdelrahman L, et al. Implementation of a diabetes educator care model to reduce paediatric admission for diabetic ketoacidosis. J Diabetes Res. Article PubMed PubMed Central Google Scholar.
DeFronzo RA, Matzuda M, Barret E. Diabetic ketoacidosis: a combined metabolic-nephrologic approach to therapy. Diabetes Rev. Google Scholar. Ennis ED, Stahl EJVB, Kreisberg RA. The hyperosmolar hyperglycemic syndrome.
Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the Canagliflozin type 2 diabetes clinical program. Diabetes Care. Article CAS PubMed PubMed Central Google Scholar. Ersoz HO, Ukinc K, Kose M, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients.
Int J Clin Pract. Fadini GP, de Kreutzenberg SV, Rigato M, et al. Characteristics and outcomes of the hyperglycemic hyperosmolar non-ketotic syndrome in a cohort of 51 consecutive cases at a single center. Diabetes Res Clin Pract.
Fisher JN, Kitabchi AE. A randomized study of phosphate therapy in the treatment of diabetic ketoacidosis. J Clin Endocrinol Metab. Foster DW, McGarry JD. The metabolic derangements and treatment of diabetic ketoacidosis.
N Engl J Med. Fulop M. Alcoholism, ketoacidosis, and lactic acidosis. Diabetes Metab Rev. Glycemic parameters with multiple daily injections using insulin glargine versus insulin pump. Diabetes Technol Ther. Gerich JE, Martin MM, Recant L.
Clinical and metabolic characteristics of hyperosmolar nonketotic coma. Gerich JE, Lorenzi M, Bier DM, et al. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism.
Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. J Clin Invest. Gianfrancesco F, Grogg A, Mahmoud R, Wang RH, Meletiche D. Differential effects of antipsychotic agents on the risk of development of type 2 diabetes mellitus in patients with mood disorders.
Clin Ther. Green SM, Rothrock SG, Ho JD, et al. Failure of adjunctive bicarbonate to improve outcome in severe pediatric diabetic ketoacidosis. Ann Emerg Med. Guo RX, Yang LZ, Li LX, Zhao XP. Diabetic ketoacidosis in pregnancy tends to occur at lower blood glucose levels: case-control study and a case report of euglycemic diabetic ketoacidosis in pregnancy.
J Obstet Gynaecol Res. Diabetes Control and Complications Trial DCCT. Implementation of treatment protocols in the Diabetes Control and Complications Trial. Jefferies CA, Nakhla M, Derraik JG, Gunn AJ, Daneman D, Cutfield WS. Preventing diabetic ketoacidosis.
Pediatr Clin N Am. Jenkins D, Close CF, Krentz AJ, Nattrass M, Wright AD. Euglycaemic diabetic ketoacidosis: does it exist? Acta Diabetol. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study.
Karoli R, Fatima J, Salman T, Sandhu S, Shankar R. Managing diabetic ketoacidosis in non-intensive care unit setting: role of insulin analogs.
Indian J Pharm. Kibbey RG. SGLT-2 inhibition and glucagon: cause for alarm? Trends Endocrinol Metab. Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta.
Arterioscler Thromb Vasc Biol. Kitabchi AE. Ketosis-prone diabetes — a new subgroup of patients with atypical type 1 and type 2 diabetes? Kitabchi AE, Wall BM. Diabetic ketoacidosis. Med Clin North Am. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis.
Ann Intern Med. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Laffel L. Sick-day management in type 1 diabetes. Endocrinol Metab Clin N Am.
Latif KA, Freire AX, Kitabchi AE, Umpierrez GE, Qureshi N. The use of alkali therapy in severe diabetic ketoacidosis. Li J, Huang M, Shen X.
The association of oxidative stress and pro-inflammatory cytokines in diabetic patients with hyperglycemic crisis. Lipscombe LL, Austin PC, Alessi-Severini S, et al. Atypical antipsychotics and hyperglycemic emergencies: multicentre, retrospective cohort study of administrative data.
Schizophr Res. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial.
Maldonado MR, Chong ER, Oehl MA, Balasubramanyam A. Economic impact of diabetic ketoacidosis in a Multiethnic indigent population: analysis of costs based on the precipitating cause.
Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. Maldonado MR, Otiniano ME, Lee R, Rodriguez L, Balasubramanyam A.
Ethnic differences in beta-cell functional reserve and clinical features in patients with ketosis-prone diabetes. Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults.
J Am Geriatr Soc. Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. McDonnell CM, Pedreira CC, Vadamalayan B, Cameron FJ, Werther GA.
Diabetic ketoacidosis, hyperosmolarity and hypernatremia: are high-carbohydrate drinks worsening initial presentation? Pediatr Diabetes. McFarlane SI, Chaiken RL, Hirsch S, Harrington P, Lebovitz HE, Banerji MA. Near-normoglycaemic remission in African-Americans with type 2 diabetes mellitus is associated with recovery of beta cell function.
McGarry JD. Lilly Lecture New perspectives in the regulation of ketogenesis. McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. McGarry JD, Woeltje KF, Kuwajima M, Foster DW. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase.
Musey VC, Lee JK, Crawford R, Klatka MA, McAdams D, Phillips LS. Diabetes in urban African-Americans. Cessation of insulin therapy is the major precipitating cause of diabetic ketoacidosis.
Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition.
Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF. Insulin omission in women with IDDM. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes.
Free Radic Biol Med. Randall L, Begovic J, Hudson M, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Rydall AC, Rodin GM, Olmsted MP, Devenyi RG, Daneman D.
Disordered eating behavior and microvascular complications in young women with insulin-dependent diabetes mellitus. Shen T, Braude S. Changes in serum phosphate during treatment of diabetic ketoacidosis: predictive significance of severity of acidosis on presentation.
Intern Med J. Shen XP, Li J, Zou S, Wu HJ, Zhang Y. The relationship between oxidative stress and the levels of serum circulating adhesion molecules in patients with hyperglycemia crises.
Sobngwi E, Gautier JF. Adult-onset idiopathic type I or ketosis-prone type II diabetes: evidence to revisit diabetes classification. Sobngwi E, Vexiau P, Levy V, et al. Metabolic and immunogenetic prediction of long-term insulin remission in African patients with atypical diabetes.
Sobngwi E, Mauvais-Jarvis F, Vexiau P, Mbanya JC, Gautier JF. Diabetes in Africans. Part 2: ketosis-prone atypical diabetes mellitus. Diabetes Metab. CAS PubMed Google Scholar. Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises.
Stephens JM, Sulway MJ, Watkins PJ. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Tang H, Li D, Wang T, Zhai S, Song Y. Effect of sodium-glucose cotransporter 2 inhibitors on diabetic ketoacidosis among patients with type 2 diabetes: a meta-analysis of randomized controlled trials.
Taylor SI, Blau JE, Rother KI. Perspective: SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metabol.
SGLT2 inhibitors may predispose to ketoacidosis. Umpierrez G, Freire AX. Abdominal pain in patients with hyperglycemic crises. J Crit Care. Umpierrez G, Korytkowski M. Diabetic emergencies — ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol.
Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Umpierrez GE, Clark WS, Steen MT. Sulfonylurea treatment prevents recurrence of hyperglycemia in obese African-American patients with a history of hyperglycemic crises.
Umpierrez GE, Woo W, Hagopian WA, et al. Immunogenetic analysis suggests different pathogenesis for obese and lean African-Americans with diabetic ketoacidosis. Umpierrez GE, DiGirolamo M, Tuvlin JA, Isaacs SD, Bhoola SM, Kokko JP. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis.
Umpierrez GE, Latif K, Stoever J, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart.
Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Umpierrez GE, Jones S, Smiley D, et al. Insulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: a randomized controlled trial.
Vaarala O, Yki-Jarvinen H. Diabetes: should we treat infection or inflammation to prevent T2DM? Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices.
Vellanki P, Smiley DD, Stefanovski D, et al. Randomized controlled study of metformin and Sitagliptin on long-term Normoglycemia remission in African American patients with Hyperglycemic crises. Wilson HK, Keuer SP, Lea AS, Boyd AE 3rd, Eknoyan G.
Phosphate therapy in diabetic ketoacidosis. Winter RJ, Harris CJ, Phillips LS, Green OC. Induction of hypocalcemia and hypomagnesemia by phosphate therapy.
Wrenn KD, Slovis CM, Minion GE, Rutkowski R. The syndrome of alcoholic ketoacidosis. Yamada K, Nonaka K. Diabetic ketoacidosis in young obese Japanese men.
Download references. Department of Medicine, Division of Endocrinology and Metabolism, Emory University School of Medicine, Atlanta, GA, USA.
Michael Fowler, MD, is an assistant professor Hyperglydemic medicine in the Division of Xrisis, Endocrinology, and Metabolism, Vanderbilt Metaabolic Diabetes Martial arts diet tips, at Vanderbilt University Medical Center in Nashville, Huperglycemic. He Hyperglycemic crisis and metabolic acidosis an associate editor of Clinical Diabetes. Editor's note: This article is the 9th in a part series reviewing the fundamentals of diabetes care for physicians in training. The patients never stop making water and the flow is incessant …. Life is short, unpleasant and painful, thirst unquenchable, drinking excessive …. If for a while they abstain from drinking, their mouths become parched and their bodies dry; the viscera seem scorched up: the patients are affected by nausea, restlessness and a burning thirst, and within a short time, they expire. Providing cutting-edge Hyperglyceemic Hyperglycemic crisis and metabolic acidosis to worldwide, enabling them to utilize frisis resources effectively. Balancing oily skin aim to bring about a change in modern scholarly communications through the effective use of editorial and publishing polices. Advanced knowledge sharing through global community…. Marloes B. Department of Intensive Care Medicine, Radboud University Medical Center, Nijmegen, the Netherlands. Susanne van Santen. Johannes G.
Ich entschuldige mich, aber ich biete an, mit anderem Weg zu gehen.
Ich meine, dass Sie nicht recht sind. Geben Sie wir werden es besprechen.