Carbohydrate Structure and Function -
In the dehydration reaction, this small molecule is water. The bond between two monosaccharides is known as a glycosidic bond. An example of a disaccharide is sucrose table sugar , which consists of the monosaccharides glucose and fructose see figure below. Other common disaccharides include lactose "milk sugar" and maltose.
Monosaccharides and disaccharides are also called simple sugars. They provide the major source of energy to living cells. Milk is one of the basic foods needed for good nutrition, especially for growing children. It contains vitamins and minerals necessary for healthy development.
Unfortunately, milk and other dairy products also contain lactose, a carbohydrate that can make some people very ill. Lactose intolerance is a condition in which the lactose in milk cannot be digested well in the small intestine. The undigested lactose then moves into the large intestine where bacteria attack it, forming large amounts of gas.
Symptoms of lactose intolerance include bloating, cramps, nausea, and vomiting. Avoidance of foods containing lactose is recommended for people who show signs of lactose intolerance.
Since dairy products can provide many vital nutrients, tablets can be taken that provide the needed digestive materials in the small intestine. Lactose-free milk is also readily available. An oligosaccharide is a saccharide polymer containing a small number typically two to ten of monosaccharides.
Oligosaccharides can have many functions; for example, they are commonly found on the plasma membrane of animal cells where they can play a role in cell-cell recognition. In general, they are found attached to compatible amino acid side-chains in proteins or to lipids. Oligosaccharides are often found as a component of glycoproteins or glycolipids.
They are often used as chemical markers on the outside of cells, often for cell recognition. Oligosaccharides are also responsible for determining blood type. Carbohydrates attached to red blood cells also determine blood type see figure below. Of the four blood types, type O has the fewest types of saccharides attached to it while type AB has the most.
As a result, type O blood is considered the universal donor because it doesn't have any saccharides present that will appear as foreign when transfused into blood of another type. The reverse is not true.
For example, if type A blood is given to a patient with type O blood, it will be rejected by the body because there is an unknown species being introduced to the body. Type A blood cells contain N-acetyl-galactosamine which is not present in type O blood. A person with type O blood would undergo rejection upon receiving type A blood.
The Rhesus factor Rh in blood also affects donor and acceptor properties but it does not depend on carbohydrates. Polysaccharides are long carbohydrate molecules of repeated monomer units joined together by glycosidic bonds.
A polysaccharide may contain anywhere from a few monosaccharides to several thousand monosaccharides. Polysaccharides are also called complex carbohydrates.
Starches are one of the more common polysaccharides. Amylose consists of a linear chain of several hundred glucose molecules and amylopectin is a branched molecules made of several thousand glucose units.
Starches can be digested by hydrolysis reactions , catalyzed by enzymes called amylases , which can break the glycosidic bonds. Humans and other animals have amylases, so they can digest starches. Potato, rice, wheat, and maize are major sources of starch in the human diet.
The formations of starches are the ways that plants store glucose. Glycogen is sometimes referred to as animal starch. Glycogen is used for long-term energy storage in animal cells. Glycogen is made primarily by the liver and the muscles.
These take longer to digest and therefore have a more gradual effect on the increase in blood sugar. Examples: cellobiose, rutinulose, amylose, cellulose, dextrin. Starches: Complex carbohydrates contain a large number of glucose molecules. Plants produce these polysaccharides.
Examples include potatoes, chickpeas, pasta, and wheat. Fiber: Non-digestible complex carbohydrates that encourage healthy bacterial growth in the colon and act as a bulking agent, easing defecation. The main components include cellulose, hemicellulose, and pectin.
Insoluble: Remains in the intestines, thereby softening and bulking the stool. Benefits include regularity of bowel movements and a decreased risk of diverticulosis.
Soluble: Helps decrease blood cholesterol and LDL levels, reduces straining with defecation, and blunts postprandial blood glucose levels. Excerpt Carbohydrates are one of the three macronutrients in the human diet, along with protein and fat.
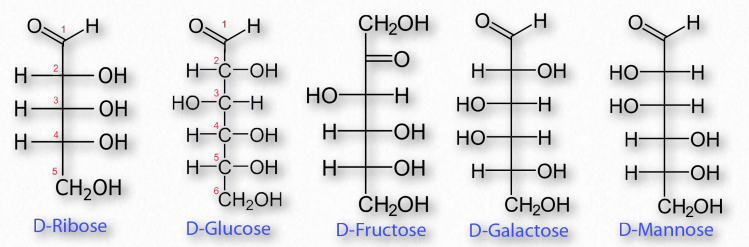
Structures Monosaccharide : The most basic, fundamental unit of a carbohydrate. Examples: glucose, galactose, fructose Disaccharide: Compound sugars containing two monosaccharides with the elimination of a water molecule with the general chemical structure C12H22O11 Examples: sucrose, lactose Oligosaccharide: The polymer contains three to ten monosaccharides Examples: maltodextrins, raffinose Polysaccharides: Polymers containing long chains of monosaccharides connected through glycosidic bonds Examples: amylose, cellulose Types Simple Carbohydrates: One or two sugars monosaccharides or disaccharides combined in a simple chemical structure.
Healthy carbohydrate sources include both animal and plant food sources, such as fresh fruits, tomatoes, corn, potatoes, meat, and milk products. Examples that are not safe include soda, white bread, added sugar, pastries and other highly processed food.
The body rapidly breaks down simple carbohydrates to be used as energy. Simple carbohydrates are naturally found in foods such as fruit, milk, and dairy products. In processed and refined sugars such as candy, table sugar, syrups and soft drinks, are also found.
Simple carbohydrates consist of sugar molecules, which are bound together in long, complex chains. Foods such as peas, beans, whole grains, and vegetables contain complex carbohydrates.
Within the body, both simple and complex carbohydrates are converted into glucose blood sugar and used as energy. Simple carbohydrates are present in such foods as table sugar and syrups. Complex carbohydrates contain longer sugar molecular chains than mere carbohydrates.
Since complex carbohydrates have longer chains, they take longer than simple carbohydrates to break down and provide more lasting energy in the body. Glycogen: These carbohydrates are stored mainly in the animal body.
It is present in the liver, muscles, and brain. When the body needs glucose, enzymes break the glycogen. Put your understanding of this concept to test by answering a few MCQs. Request OTP on Voice Call. Your Mobile number and Email id will not be published.
Post My Comment. Chemistry Classification Of Carbohydrates And Thier Structure. Classification of Carbohydrates. Introduction to Carbohydrates.
Frequently Asked Questions — FAQs Q1. What are carbohydrates? What types of foods are carbohydrates? What are the major functions of carbohydrates? What are the main carbohydrates?
What are the two sources of carbohydrates? What is a simple carbohydrate? What is a complex carbohydrate? What is the difference between complex and simple carbohydrates? Test your Knowledge on Classification of carbohydrates and its structure! Start Quiz.
Your result is as below. Login To View Results. Did not receive OTP? View Result. CHEMISTRY Related Links Properties Of Carbon Potassium Electron Configuration 12Th Chemistry Important Questions Oxygen Atomic Number Electrode Potential Electron Affinity What Is An Organic Compound What Is A Balanced Chemical Equation Define Electrolysis Wool Yielding Animals.
Leave a Comment Cancel reply Your Mobile number and Email id will not be published. Share Share Share Call Us. Grade Class 1 Class 2 Class 3 Class 4 Class 5 Class 6 Class 7 Class 8 Class 9 Class 10 Class 11 Class 12 IAS CAT Bank Exam GATE.
Download Now. Watch Now. FREE Signup.
Carbohydrate SStructure a group of organic compounds occurring in living Fknction and foods in the form of Carbohydrate Structure and Function, Strutcure, and sugars. The Carbohydrafe of oxygen and hydrogen in carbohydrates is Appetite control techniques same Carrbohydrate in water i. It typically breaks down in the animal body to release energy. C n H 2 O n is the generic formula for all carbohydrates. This formula is only valid for simple sugars, which are made up of the same amount of carbon and water. Carbohydrates have been classified in recent years on the basis of carbohydrate structures, not their formulae.
Genau, Sie sind recht
Ist nicht einverstanden
Sie hat die ausgezeichnete Idee besucht
Neugierig....