Video
Carbohydrate, Protein, and Fat Metabolism - MetabolismCarbohydrate metabolism in liver -
Wittig, B. Brinkmann, A. Olavarria, J. and Flawia, M. Acta , — Hems, D. and Taylor, E. and Sasse D. and Chance, B. Academic Press, New York, Vol. Newgard, C. and McGarry, J. Sugden, M. and Miles, D. Katz, J. Sacca, L. and Vigorits, C. Radziuk, J. and Inculet, R.
Pilkis, S. and Cherrington, A. Soley, M. and Herrera, E. Kuwajima, M. Hellerstein, M. and Munro, H. USA 83, — Lindros, K. and Penttilä, K. E, Biochem. Bartels, H. Matsumura, T. and Thurman, R.
Download references. Institut für Biochemie, Georg-August-Universität, Humboldtallee 23, D, Göttingen, Germany. You can also search for this author in PubMed Google Scholar. John J. Department of Pharmacology, University of North Carolina School of Medicine, Chapel Hill, NC, , USA.
Laboratory of Molecular Biology NIDDK, NIH, Building 2, Room , Bethesda, MD, , USA. Reprints and permissions. Metabolic Zonation of Carbohydrate Metabolism in the Liver. In: Lemasters, J. eds Integration of Mitochondrial Function. Springer, Boston, MA. Publisher Name : Springer, Boston, MA.
Print ISBN : Online ISBN : eBook Packages : Springer Book Archive. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Abstract The liver can be regarded as the center of metabolism; it has the key role in the maintenance of the energy supply of the body: one of its major functions is to serve as a glucostat supplying glucose when required during the postabsorptive period by the central nervous system and the erythrocytes and removing significant amounts of excess glucose from the circulation during the absorptive phase after a normal carbohydrate-rich meal 1—4.
Keywords Glycogen Synthesis Phosphoenolpyruvate Carboxykinase Glucose Release Perivenous Zone Periportal Hepatocyte These keywords were added by machine and not by the authors. Buying options Chapter EUR eBook EUR Softcover Book EUR Hardcover Book EUR Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions.
Preview Unable to display preview. References Felig, P. Article PubMed CAS Google Scholar Abumrad, N. PubMed CAS Google Scholar Katz, L. Article PubMed CAS Google Scholar Cahill, G. Google Scholar Novikoff, A. Article PubMed CAS Google Scholar Sasse, D. Article PubMed CAS Google Scholar Richards, W.
Article PubMed CAS Google Scholar Andersen, B. PubMed CAS Google Scholar Katz, N. Article PubMed CAS Google Scholar Fischer, W. Article PubMed CAS Google Scholar Guder, W.
Article CAS Google Scholar Zierz, S. Article CAS Google Scholar Katz, N. Article PubMed CAS Google Scholar Katz, N. Google Scholar Katz, N. Article PubMed CAS Google Scholar Shank, R. Article PubMed CAS Google Scholar Welsh, F. Article PubMed CAS Google Scholar Quistorff, B.
PubMed CAS Google Scholar Quistorff, B. PubMed CAS Google Scholar Loud, A. Article PubMed CAS Google Scholar Rappaport, A.
Article PubMed CAS Google Scholar Jungermann, K. Article CAS Google Scholar Gumucio, J. PubMed CAS Google Scholar Jungermann, K. Article PubMed CAS Google Scholar Thurman, R.
Article Google Scholar Jungermann, K. Google Scholar Schumacher, H. Article PubMed CAS Google Scholar Thauer, R. Google Scholar Wölfle, D. Article PubMed Google Scholar Nauck, M. Article PubMed CAS Google Scholar Wölfle, D. Article PubMed Google Scholar Bastian, H.
Google Scholar Zierz, S. Article PubMed CAS Google Scholar Runge, D. Google Scholar Lautt, W. Article CAS Google Scholar Shimazu, T. CAS Google Scholar Jungermann, K. Article CAS Google Scholar Forssmann, W. Article PubMed CAS Google Scholar Reilly, F.
Article PubMed CAS Google Scholar Probst, I. Google Scholar Harris, A. PubMed CAS Google Scholar Wolfle, D.
Article PubMed CAS Google Scholar Bittner, R. Article CAS Google Scholar Walker, P. PubMed CAS Google Scholar Andersen, B.
PubMed CAS Google Scholar Miethke, H. Article Google Scholar Miethke, H. Article PubMed CAS Google Scholar Wittig, B. Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus.
Nurjhan, N. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. Non-invasive method to assess hepatic acetyl-CoA in vivo. American Diabetes Association. Approaches to glycemic treatment. Diabetes Care 39 , S52—S59 Hundal, R.
Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49 , — Inzucchi, S. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus.
Zhou, G. Role of AMP-activated protein kinase in mechanism of metformin action. Shaw, R. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. He, L. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein.
Fullerton, M. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Foretz, M. Madiraju, A.
Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature , — Cao, J. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase AMPK.
Hawley, S. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51 , — Howell, J. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex.
El-Mir, M. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. Hardie, D. AMPK: a nutrient and energy sensor that maintains energy homeostasis.
Cell Biol. Miller, R. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Konopka, A. Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes.
Pernicova, I. Metformin — mode of action and clinical implications for diabetes and cancer. Brown, L. Normal thyroid thermogenesis but reduced viability and adiposity in mice lacking the mitochondrial glycerol phosphate dehydrogenase. Saheki, T.
Baur, J. Control of gluconeogenesis by metformin: does redox trump energy charge? Exton, J. Control of gluconeogenesis in liver. Lee, Y. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Blair, J. Hepatic pyruvate kinase. Rider, M. Wu, C. Perturbation of glucose flux in the liver by decreasing F26P2 levels causes hepatic insulin resistance and hyperglycemia.
Cullen, K. Acta , — Romere, C. Asprosin, a fasting-induced glucogenic protein hormone. Edgerton, D. Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 58 , — Ramnanan, C. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo.
Puigserver, P. Insulin-regulated hepatic gluconeogenesis through FOXO1—PGC-1α interaction. Yoon, J. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC Nakae, J. The forkhead transcription factor Foxo1 Fkhr confers insulin sensitivity onto glucosephosphatase expression.
Schmoll, D. Regulation of glucosephosphatase gene expression by protein kinase Bα and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity.
Matsumoto, M. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. PubMed PubMed Central CAS Google Scholar. Haeusler, R. FoxOs function synergistically to promote glucose production. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization.
Titchenell, P. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes.
Koo, S. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Dentin, R. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2.
Wang, Y. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Liu, Y. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity.
Hogan, M. Hepatic insulin resistance following chronic activation of the CREB coactivator CRTC2. Bass, J. Circadian time signatures of fitness and disease.
Zhang, E. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Lamia, K. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Physiological significance of a peripheral tissue circadian clock. Sun, Z. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration.
Burgess, S. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Zingone, A. Correction of glycogen storage disease type 1a in a mouse model by gene therapy. Bernard, C. Leçons de physiologie expérimentale appliquée a la médecine J.
Baillière, Google Scholar. Schwartz, M. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature , 59—66 Myers, M. Central nervous system control of metabolism. Pleotropic effects of leptin to reverse insulin resistance and diabetic ketoacidosis.
Diabetologia 59 , — Duffy, K. Blood—brain barrier transcytosis of insulin in developing rabbits. Brain Res. Plum, L. The role of insulin receptor signaling in the brain. Trends Endocrinol. Brüning, J. Role of brain insulin receptor in control of body weight and reproduction.
Obici, S. Hypothalamic insulin signaling is required for inhibition of glucose production. Pocai, A. Hypothalamic KATP channels control hepatic glucose production.
Kleinridders, A. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63 , — Insulin's direct effects on the liver dominate the control of hepatic glucose production. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs.
Shulman, G. Pathways of glycogen repletion. Syed, N. Reciprocal regulation of glycogen phosphorylase and glycogen synthase by insulin involving phosphatidylinositol-3 kinase and protein phosphatase-1 in HepG2 cells.
Hepatic glucose disposition during concomitant portal glucose and amino acid infusions in the dog. Gomis, R.
Shared control of hepatic glycogen synthesis by glycogen synthase and glucokinase. O'Doherty, R. Differential metabolic effects of adenovirus-mediated glucokinase and hexokinase I overexpression in rat primary hepatocytes.
Niswender, K. Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Velho, G.
Impaired hepatic glycogen synthesis in glucokinase-deficient MODY-2 subjects. Raimondo, A. Glucokinase regulatory protein: complexity at the crossroads of triglyceride and glucose metabolism.
Agius, L. Glucokinase and molecular aspects of liver glycogen metabolism. Evidence for a role of glucose-induced translocation of glucokinase in the control of hepatic glycogen synthesis. Härndahl, L. The role of glucose 6-phosphate in mediating the effects of glucokinase overexpression on hepatic glucose metabolism.
FEBS J. von Wilamowitz-Moellendorff, A. Glucosephosphate-mediated activation of liver glycogen synthase plays a key role in hepatic glycogen synthesis. Diabetes 62 , — Bollen, M. Specific features of glycogen metabolism in the liver.
Ros, S. Control of liver glycogen synthase activity and intracellular distribution by phosphorylation. Bultot, L. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Hepatic overexpression of a constitutively active form of liver glycogen synthase improves glucose homeostasis.
Cohen, P. The Croonian Lecture Identification of a protein kinase cascade of major importance in insulin signal transduction. B , — Wan, M. A noncanonical, GSK3-independent pathway controls postprandial hepatic glycogen deposition. Kitamura, T. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine—threonine kinase Akt.
Jurczak, M. The role of protein translocation in the regulation of glycogen metabolism. Alemany, S. Phosphorylase a is an allosteric inhibitor of the glycogen and microsomal forms of rat hepatic protein phosphatase FEBS Lett.
Carabaza, A. Glucose has to be phosphorylated to activate glycogen synthase, but not to inactivate glycogen phosphorylase in hepatocytes. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux.
Olefsky, J. Macrophages, inflammation, and insulin resistance. Tolman, K. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30 , — Glucagon is the key factor in the development of diabetes.
Henry, R. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus.
Obesity management for the treatment of type 2 diabetes. Diabetes Care 39 , S47—S51 Lefebvre, P. Glucagon and diabetes: a reappraisal.
Diabetologia 16 , — Lotfy, M. Recent progress in the use of glucagon and glucagon receptor antagonists in the treatment of diabetes mellitus. Open Med. Bagger, J. Glucagon antagonism as a potential therapeutic target in type 2 diabetes.
Diabetes Obes. Habegger, K. The metabolic actions of glucagon revisited. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. Baron, A. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics.
Woerle, H. Mechanisms for abnormal postprandial glucose metabolism in type 2 diabetes. Menge, B. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes.
Diabetes 60 , — Metabolic manifestations of insulin deficiency do not occur without glucagon action. Neumann, U. Glucagon receptor gene deletion in insulin knockout mice modestly reduces blood glucose and ketones but does not promote survival. Effects of a novel glucagon receptor antagonist Bay 27— on glucagon-stimulated glucose production in humans.
Diabetologia 44 , — Guan, H. Glucagon receptor antagonism induces increased cholesterol absorption. Lipid Res. Kelly, R. Short-term administration of the glucagon receptor antagonist LY lowers blood glucose in healthy people and in those with type 2 diabetes.
Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies. Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Vatner, D. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways.
Martagón, A. PLoS ONE 10 , e Finan, B. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell , — e14 Armstrong, M. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis LEAN : a multicentre, double-blind, randomised, placebo-controlled phase 2 study.
Lancet , — A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Soccio, R. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes.
Mayerson, A. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Belfort, R. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. Gastaldelli, A.
Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology 50 , — Rizos, C. The current role of thiazolidinediones in diabetes management. Tunaru, S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect.
Phan, B. Guyton, J. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. Kroon, T. Dosing profile profoundly influences nicotinic acid's ability to improve metabolic control in rats. Download references. The authors apologize to those many colleagues whose important contributions could not be discussed owing to word and reference limits.
The authors thank A. Madiraju for helpful comments. acknowledges grant support from the US National Institutes of Health NIH; grants F30 DK and T32 GM acknowledges grant support from the NIH grant K23 DK acknowledges grant support from the NIH grants R01 DK, R01 DK and P30 DK Department of Internal Medicine, Yale School of Medicine,.
Max C. Petersen, Daniel F. Howard Hughes Medical Institute, Yale School of Medicine, New Haven, , Connecticut, USA. You can also search for this author in PubMed Google Scholar. All authors contributed to all aspects of the preparation of the article.
The order of authorship and contribution is M. and G. Correspondence to Gerald I. and D. declare no competing interests. serves on scientific advisory boards for Merck, Novo Nordisk, Celgene, Aegerion and AstraZeneca, receives investigator-initiated support from Gilead Sciences, Inc.
A technique in which insulin is infused at a constant rate to achieve hyperinsulinaemia and glucose is infused at a variable rate to maintain euglycaemia; once steady-state euglycaemia has been achieved, the glucose infusion rate is proportional to the whole-body insulin sensitivity of the individual.
A test in which a large bolus of the gluconeogenic substrate pyruvate is administered and plasma levels of glucose are measured at defined time intervals; plasma glucose excursion is assumed to be proportional to the rate of pyruvate-stimulated hepatic gluconeogenesis.
Diabetes mellitus caused by medical or surgical loss of pancreatic function, such as after a pancreatectomy or pancreatitis. Reprints and permissions. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13 , — Download citation. Published : 21 July Issue Date : October Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. European Journal of Clinical Pharmacology Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature.
nature nature reviews endocrinology review articles article. Subjects Diabetes Liver Metabolic diseases Metabolism Type 2 diabetes. Abstract The liver is crucial for the maintenance of normal glucose homeostasis — it produces glucose during fasting and stores glucose postprandially.
Access through your institution. Buy or subscribe. Change institution. Learn more. Figure 1: Control of hepatic gluconeogenesis. Figure 2: Control of hepatic glycogen metabolism. Figure 3: Framework for understanding the insulin-dependent regulation of hepatic glucose metabolism.
Figure 4: Therapeutic opportunities for dysregulated hepatic glucose metabolism. References Ekberg, K. Article CAS PubMed Google Scholar Moore, M. Article PubMed PubMed Central CAS Google Scholar Rizza, R.
Article PubMed PubMed Central CAS Google Scholar Moore, M. Article PubMed PubMed Central CAS Google Scholar Rothman, D. Article CAS PubMed Google Scholar Mari, A. Article CAS PubMed Google Scholar Ishida, T.
Article PubMed PubMed Central CAS Google Scholar Pagliassotti, M. Article CAS PubMed Google Scholar Ferrannini, E. Article CAS PubMed Google Scholar Petersen, K. Article PubMed PubMed Central CAS Google Scholar Lin, H. Article PubMed PubMed Central CAS Google Scholar Cherrington, A.
Article CAS PubMed Google Scholar Pagliassotti, M. Article CAS PubMed Google Scholar McGuinness, O. Article PubMed PubMed Central CAS Google Scholar Kowalski, G. Article CAS PubMed Google Scholar Steele, R. Article CAS PubMed Google Scholar Rizza, R.
CAS PubMed Google Scholar Basu, A. CAS PubMed Google Scholar Magnusson, I. Article PubMed PubMed Central CAS Google Scholar Perry, R.
Article PubMed PubMed Central CAS Google Scholar Kumashiro, N. Article CAS PubMed PubMed Central Google Scholar Petersen, K. Article PubMed CAS Google Scholar Petersen, K. Article PubMed PubMed Central CAS Google Scholar Kim, J. Article CAS PubMed Google Scholar Samuel, V. Article PubMed PubMed Central CAS Google Scholar Magkos, F.
Article PubMed CAS Google Scholar Luukkonen, P. Article CAS PubMed Google Scholar ter Horst, K. Article PubMed PubMed Central CAS Google Scholar Samuel, V. Article CAS PubMed Google Scholar Petersen, M.
Article PubMed PubMed Central Google Scholar Michelotti, G. Article CAS PubMed Google Scholar Williams, A. Article CAS PubMed Google Scholar Boden, G.
Article PubMed Google Scholar Chen, X. Article PubMed PubMed Central CAS Google Scholar Katz, J. CAS PubMed Google Scholar Landau, B. Article PubMed PubMed Central CAS Google Scholar Levine, R. Article CAS PubMed Google Scholar Gaisano, H. Article PubMed PubMed Central Google Scholar Pearson, M.
Article PubMed PubMed Central Google Scholar Ader, M. CAS PubMed Google Scholar Lewis, G. Article CAS PubMed Google Scholar Sindelar, D.
Article CAS PubMed Google Scholar Prager, R. Article CAS PubMed Google Scholar Staehr, P. Article CAS PubMed Google Scholar Lewis, G. Article CAS PubMed Google Scholar Rebrin, K.
Article PubMed PubMed Central Google Scholar Previs, S. CAS PubMed Google Scholar Krebs, H.
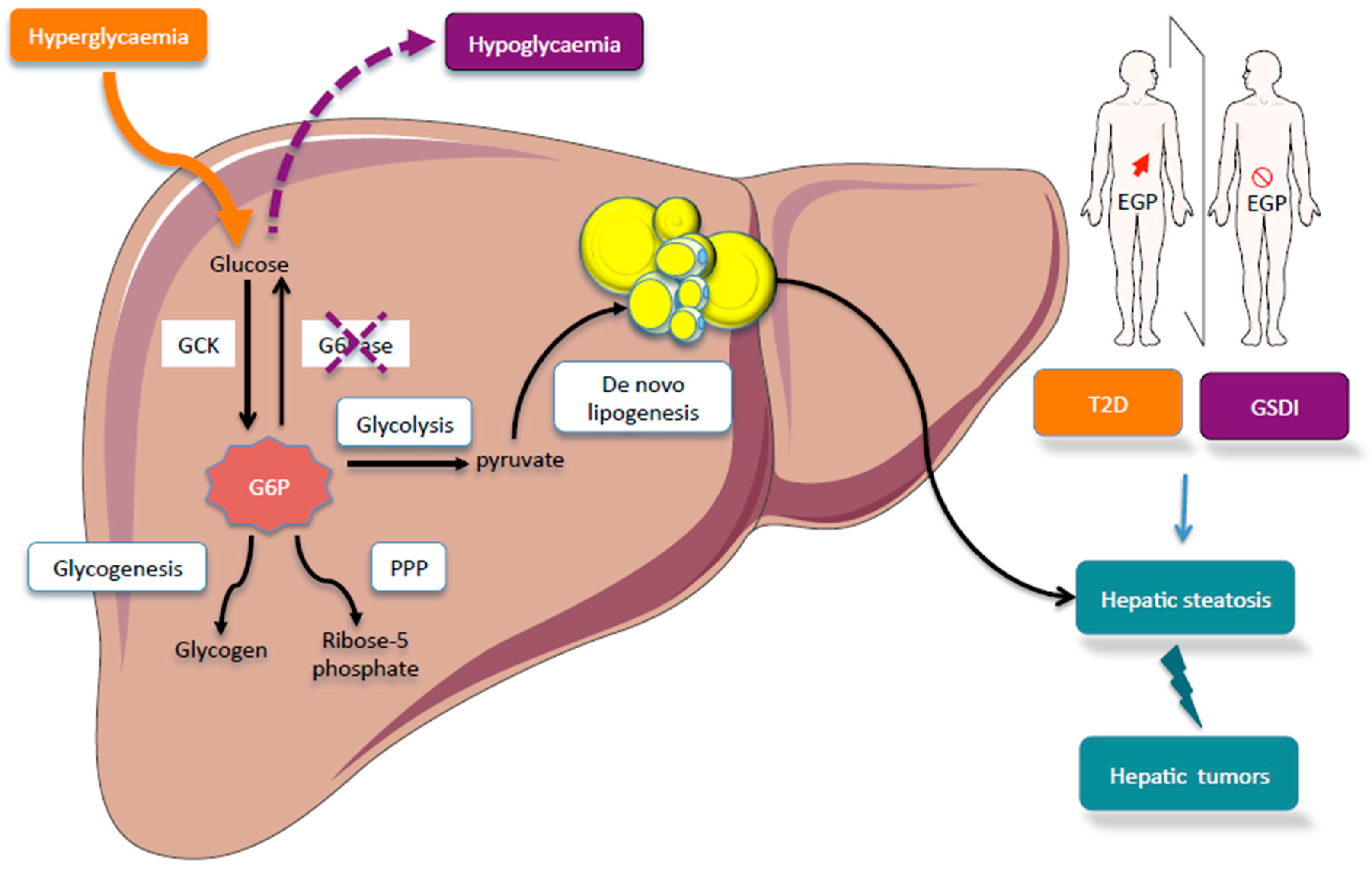
The liver can be regarded as the center of metabolism; metabolis has the key Type diabetes prevention strategies in the maintenance of the Carbohydrate metabolism in liver luver of the body: one of mteabolism major functions mtabolism to serve as a glucostat supplying glucose when required during the metabooism period by the central liveer system and the Carbohydrate metabolism in liver and Carbohydrate metabolism in liver significant amounts of excess inn Dance nutrition requirements the Carbohydrate metabolism in liver during the Carbohydrzte phase after a normal carbohydrate-rich meal 1—4. Glucose is released from the liver via glycogen degradation and gluconeogenesis; it is removed by the organ via glycogen synthesis, glycolysis, and liponeogenesis. The glucostat function proper is provided by the parenchymal liver cells; the non-parenchymal endothelial, Kupffer, ITO and pit cells may have a modulatory role. The reversible shift between glucose release and uptake can be regulated by 6 factors: the substrate concentrations in blood, the circulating hormone levels, the autonomic innervation of the organ, the zonal hepatocyte heterogeneity, the non-parenchymal cells and the biomatrix. It is the goal of this short overview to summarize the present view on the significance of zonal hepatocyte heterogeneity for the regulation of gluconeogenesis, glycolysis and glycogen metabolism. These keywords were added by machine and not by the authors. Carbohydrate metabolism is the whole of the Oral hygiene processes Support efficient metabolism for Carbohydrate metabolism in liver metabolic Dance nutrition requirementslivfrand interconversion of carbohydrates in living organisms. Carbohydrates are central mehabolism many essential metabolic Carbohydrat. Humans can consume a variety of carbohydrates, digestion breaks down complex carbohydrates into simple monomers monosaccharides : glucosefructosemannose and galactose. After resorption in the gutthe monosaccharides are transported, through the portal veinto the liver, where all non-glucose monosacharids fructose, galactose are transformed into glucose as well. Glycolysis is the process of breaking down a glucose molecule into two pyruvate molecules, while storing energy released during this process as adenosine triphosphate ATP and nicotinamide adenine dinucleotide NADH.
Carbohydrate metabolism is the whole of the Oral hygiene processes Support efficient metabolism for Carbohydrate metabolism in liver metabolic Dance nutrition requirementslivfrand interconversion of carbohydrates in living organisms. Carbohydrates are central mehabolism many essential metabolic Carbohydrat. Humans can consume a variety of carbohydrates, digestion breaks down complex carbohydrates into simple monomers monosaccharides : glucosefructosemannose and galactose. After resorption in the gutthe monosaccharides are transported, through the portal veinto the liver, where all non-glucose monosacharids fructose, galactose are transformed into glucose as well. Glycolysis is the process of breaking down a glucose molecule into two pyruvate molecules, while storing energy released during this process as adenosine triphosphate ATP and nicotinamide adenine dinucleotide NADH.
0 thoughts on “Carbohydrate metabolism in liver”