
Arthritis medication side effects -
Read more about specific medication side effects in our Rheumatoid Arthritis Drug Side Effects Chart. Drug manufacturers are required to inform patients about all the possible risks that are associated with a drug — including those that happen rarely.
The side effects that manufacturers warn you about primarily stem from what occurred in clinical trials that preceded a drug being approved by the U. Food and Drug Administration FDA. Post-marketing studies which are done after a drug hits the market may also reveal new side effects over time.
The FDA also maintains an Adverse Event Reporting System that enables physicians, patients, and manufacturers to submit any perceived safety issues to the FDA. Side effects that are reported through that system may prompt the FDA to require the manufacturer to add additional warnings to a drug or possibly even pull it from the market.
Drug package inserts and advertisements sometimes distinguish between side effects that are rare and those that are more likely to occur, but not always. Your age, sex, and other health problems you might have such as diabetes, heart disease, etc.
are all important factors that could raise or lower your risk of experiencing drug side effects. In general, Dr. You should always talk to your doctor about your personal health history and lifestyle, and how those could affect your risk for certain drug side effects. All drugs can cause side effects, but some drugs increase the risk of extremely serious problems, including severe injury, illness, or death.
Many drugs used to treat RA carry black box warnings. This kind of warning should make you and your doctor pause and spend time discussing the risks and weighing them against the benefits that the drug might offer you before making a decision.
With this information in mind, doctors can avoid prescribing certain medications to patients who are most apt to have problems with them. For example, Dr. Winthrop explains:. How many medications you take and their dosages matters, too.
Even if you take only one drug, a lower dose may reduce the degree of side effects. Methotrexate, for instance, is often given to cancer patients in doses of to mg, says Dr.
Rheumatoid arthritis patients typically take 10 to 25 mg. When it comes to methotrexate, Dr. Dao adds that taking a daily folic acid supplement can greatly reduce your chances of experiencing side effects such as hair loss, mouth sores, and liver problems. Medication side effects can be scary.
You should always discuss them with your doctor before starting a new medication. In some cases, what you think is a side effect might really be a symptom of the disease itself, says Dr. In other instances, you might need to change your dose or switch to another medication.
But choosing not to take medication for RA is incredibly dangerous. Long-term inflammation can permanently damage your joints and raise your risk of life-threatening conditions, such as heart and lung disease.
The risk of your disease progressing without treatment is about percent. Winthrop adds that rheumatoid arthritis patients always have choices — lots of them. Says Dr. Tags: Rheumatoid Arthritis. Methotrexate is effective in reducing the signs and symptoms of RA, as well as slowing or halting radiographic damage.
It was as effective as leflunomide and sulfasalazine in one study, and its effectiveness given early and in higher doses approached the efficacy of etanercept and adalimumab as single therapies in terms of signs and symptom improvement.
Methotrexate is also effective in many other forms of inflammatory arthritis including psoriatic arthritis and other spondyloarthopathies, and is used in many other autoimmune diseases. The anti-inflammatory effects of methotrexate in rheumatoid arthritis appear to be related at least in part to interruption of adenosine and possible effects on other inflammatory and immunoregulatory pathways.
The immunosuppressive and toxic effects of methotrexate are due to the inhibition of an enzyme involved in the metabolism of folic acid, dihydrofolate reductase. Dosing typically begins at A dose escalation to 20 mg within the first three months is now fairly well accepted in clinical practice.
Maximal dose is usually 25 mg per week but is sometimes increased further to 30 mg. Methotrexate can be given orally or by subcutaneous injection. The latter route of administration can be advantageous for patients who have methotrexate-associated nausea.
Patients starting methotrexate should be carefully evaluated for renal insufficiency, acute or chronic liver disease, significant alcohol intake or alcohol abuse, leukopenia low white blood cell counts , thrombocytopenia low platelet counts , or untreated folate deficiency. Obesity, diabetes and history of hepatitis B or C are factors that have been suggested but not confirmed to increase methotrexate hepatotoxicity liver injury.
Salicylates and other NSAIDs and the antibiotic trimethoprim Bactrim®, Septra® block the renal excretion of methotrexate and increase serum levels with an increased risk of toxicity.
If alternatives exist, concomitant use of methotrexate and trimethoprim is to be avoided. The coadministration of NSAIDS with methotrexate is routine in patients with rheumatoid arthritis and is considered safe by rheumatologists as long as liver function tests and blood counts are closely monitored.
The onset of action is seen in as early as 4 to 6 weeks. However the dose required to achieve a response is variable in individual patients and may require weeks after a dose increase to determine if the drug is working.
A trial of 3 to 6 months at an increased dose e. In patients with partial responses to methotrexate, additional medications are usually added to rather than substituted for methotrexate to achieve combination therapies.
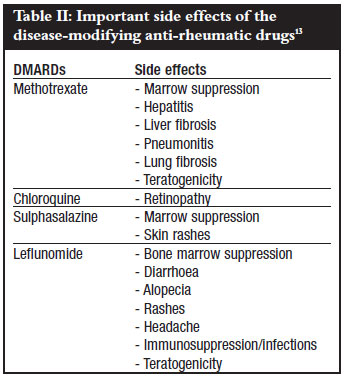
Fortunately the most serious complications of methotrexate therapy: hepatic cirrhosis, interstitial pneumonitis, and severe myelosuppression are quite rare, especially with proper monitoring. Stomatitis and oral ulcers, mild alopecia and hair thinning, and GI upset may occur and are related to folic acid antagonism.
These side effects can be improved with folic acid supplementation. Folic acid given at a dose of 1mg daily does not diminish the efficacy of methotrexate and is routinely given with methotrexate to decrease these side effects. These side effects can often be overcome by increasing folic acid or using an activated form of folic acid known as folinic acid leukovorin® given as a 5mg dose 12 hours and sometimes 24 hours after methotrexate is given.
Some patients complain of GI upset nausea or diarrhea with oral methotrexate. This may be lessened when methotrexate is taken at night. In most cases this is completely eliminated when methotrexate is given by subcutaneous administration. Before starting methotrexate, baseline studies should include complete blood count, liver chemistries, serum creatinine, hepatitis B and C serologies, and chest X-ray.
Routine toxicity monitoring should include a CBC, liver profile, serum albumin and serum creatinine every weeks. Methotrexate can be combined safely with nearly every other FDA-approved DMARDs for RA, including sulfasalazine, hydroxychloroquine, TNF inhibitors, abatacept, rituximab, tocilizumab, anakinra, and leflunomide.
In all clinical trials combining methotrexate with one of these DMARDs, no unexpected toxicities or synergistic toxicities were observed with the exception of higher liver toxicity with leflunomide which is also metabolized by the liver.
Hepatotoxicity liver injury has not been significant if patients with pre-existing liver disease, alcohol abuse, or hepatic dysfunction are excluded from treatment with methotrexate.
Patients are instructed to limit alcohol containing beverages to no more than one-two per week. Baseline or surveillance liver biopsies are not indicated unless pre-existing liver disease is suspected. Elevated liver enzymes do not directly correlate with toxicity but therapy should be stopped and doses of methotrexate reduced if transaminases are elevated to 2 times the upper limit of normal.
Liver biopsy should be done if elevated liver enzymes persist or if methotrexate therapy is to be continued. Methotrexate pneumonitis may occur at any time during therapy and is not dose related. A baseline chest x-ray is useful for comparison.
Patients with poor pulmonary reserve from other causes may be excluded from therapy over concerns of increased morbidity if methotrexate pneumonitis occurs. A more chronic form of interstitial lung disease and fibrosis is also seen in patients with rheumatoid arthritis.
This may be increased with methotrexate. Myelosuppression lowering of blood counts is also rare at the low doses of methotrexate utilized for rheumatoid arthritis. Patients at particular risk include those with renal insufficiency from other causes or use of trimethoprim Bactrim®, Septra® which increases levels of methotrexate.
In the absence of leukopenia lowered white blood cell counts , there has not been conclusive information to link methotrexate use in rheumatoid arthritis with increased risk of infection.
The exception is a slight increased risk of localized herpes zoster infection shingles. Cancer risk with methotrexate. Although there are case reports of lymphoma associated with methotrexate therapy including cases where the lymphoma resolved after cessation of therapy, increased occurrence of malignancy has not been found in large population-based studies.
It is important to recognize that patient with rheumatoid arthritis have an increased risk of developing lymphoma as a consequence of their autoimmune disease, independently from any potential medication effects. Pregnancy and Conception with methotrexate. There have not been any notable effects on sperm production or ovarian function after the prolonged administration of methotrexate.
However, methotrexate is considered a teratogen ; therefore, women of childbearing potential or men with partners of childbearing potential must practice effective birth control.
Women should discontinue methotrexate for at least one ovulatory cycle prior to attempting conception, while men should wait 3 months. Hydroxychloroquine is an antimalarial drug which is relatively safe and well-tolerated agent for the treatment of rheumatoid arthritis.
Chloroquine is another antimalarial agent that is also sometimes used. Because these drugs have limited ability to prevent joint damage on their own, their use should probably be limited to patients with very mild, seronegative, and nonerosive disease.
The mechanism of action of antimalarials in the treatment of patients with rheumatoid arthritis is unknown but is thought to involve changes in antigen presentation or effects on the innate immune system.
Dosage: Hydroxychloroquine Plaquenil® is the drug of choice among antimalarials. Chloroquine is not commonly used because of greater toxicity on the eye. It may be prescribed as a single daily dose or in divided doses twice per day.
A period of 2 to 4 months is usual. Most agree that if a patient shows no response after months that this should be considered a drug failure. The most important toxicities are on the eyes: corneal deposits, extraocular muscular weakness, loss of accommodation and sensitivity to light , and a retinopathy that may progress to irreversible visual loss.
Ocular toxicity is exceedingly rare, occurring in only 1 out of 40, patients treated at the doses recommended. Patients with underlying retinopathies or risks may not be good candidates for antimalarial drugs. Baseline ophthalmologic examination and a follow-up examination every 12 months are recommended during the period of treatment.
Sulfasalazine Azulfidine® is an effective DMARD for the treatment of RA. Its effectiveness overall is somewhat less than that methotrexate, but it has been shown to reduce signs and symptoms and slow radiographic damage.
Sulfasalazine is also used in the treatment of inflammatory bowel disease and spondyloarthropathies. Its mechanism of action in RA is unknown.
Some of its effects may be due to folate depletion. The usual dose is grams per day in a twice daily dosing regimen. The dose may be initiated at 1 gram per day and increased as tolerated.
Sulfasalazine may cause hypersensitivity and allergic reactions in patients who have experienced reactions to sulfa medications.
Mild gastrointestinal complaints are commonly seen and these can be decreased by using enteric coated formulations or administration of the medication with meals. Occasionally, mild cytopenias are seen. Patients may be screened before the use of sulfasalazine for a deficiency of the enzyme glucosephosphate dehydrogenase G6PD which may predispose patients to red blood cell hemolysis and anemia.
Blood monitoring is typically every months depending on dose. Though sulfasalazine may cause increases in liver function tests, it is generally considered a preferable agent to methotrexate in patients with liver disease or in patients who have hepatitis B or C.
Leflunomide is also an effective DMARD. Its efficacy is similar to methotrexate in terms of signs and symptoms, and is a viable alternative to patients who have failed or are intolerant to methotrexate. Leflunomide has been demonstrated to slow radiographic progression. Studies have demonstrated that it can also be carefully combined with methotrexate in patients with no preexisting liver disease, as long as the liver function tests are carefully monitored.
Leflunomide has also been studied in psoriatic arthritis with some efficacy demonstrated. The mechanism of action of leflunomide is not fully understood but may be related to its ability to inhibit de novo pyrimidine biosynthesis through the inhibition of the enzyme dihydroorotate dehydrogenase.
Laboratory studies have demonstrated that it also has effects on stimulated T cells. The half-life of the active metabolite of leflunomide is very long. Leflunomide and its metabolites are extensively protein bound and undergo further metabolism before excretion.
When initially approved, the medication was given using a loading dose of mg daily for three days then followed by 20 mg daily. The dose may be reduced to 10mg daily if not tolerated at the 20 mg dose.
The onset of action is relatively rapid within weeks. The onset of action of Arava may be seen earlier than methotrexate when using a loading dose. Leflunomide has been associated with liver transaminase elevations that reversed with cessation of the drug in clinical trials.
Routine monitoring should include complete blood count and hepatic panel more frequently at the beginning of therapy then on a regular basis at least every 2 months.
Other toxicities that are common include mild diarrhea, GI upset and alopecia and hair thinning sometimes of sufficient severity to cause cessation of the drug. Because leflunomide and its metabolites are a teratogen , extreme care must be taken for treatment of women of child bearing potential.
Women must be warned about the possible risk to the fetus and cautioned to use adequate birth control. Women wishing to become pregnant must take cholestyramine 8gm 3 times daily for 11 days and then have two leflunomide metabolite levels drawn 14 days apart to document serum concentration less than 0.
Leflunomide treatment does not appear to be associated with an increased risk for infection. Tumor necrosis factor alpha TNF is a pro-inflammatory cytokine produced by macrophages and lymphocytes. It is found in large quantities in the rheumatoid joint and is produced locally in the joint by synovial macrophages and lymphocytes infiltrating the joint synovium.
TNF is one of the critical cytokines that mediate joint damage and destruction due to its activities on many cells in the joint as well as effects on other organs and body systems. TNF antagonists were the first of the biological DMARDS to be approved for the treatment of RA. These drugs began to enter the market for rheumatoid arthritis in and are now considered a part the ACR recommendations for treatment of RA.
There are currently five TNF inhibitors FDA approved for the treatment of RA listed in order of their approval for RA ; etanercept Enbrel® , infliximab Remicade® , adalimumab Humira® , certolizumab pegol Cimzia® , and golimumab Simponi®.
Etanercept is a soluble TNF receptor-Fc immunoglobulin fusion construct; infliximab, adalimumab, and golimumab are monoclonal antibodies; and certolizumab pegol is an anti-TNF antigen binding domain-polyethylene glycol construct.
While differing in structure, the efficacy and safety of the drugs is similar across the class in reducing the signs and symptoms of RA, as well as in slowing or halting radiographic damage, when used either as monotherapy or in combination with methotrexate.
Usual Time to Effect : TNF inhibitors have a rapid onset of action sometimes with improvements seen within 2 to 4 weeks. However, additional improvements can be seen over months. Side Effects : With all TNF antagonists, there is an increased risk of infection both mild and severe.
The most common are upper respiratory infections, pneumonia, urinary tract infections, and skin infections. Studies are currently ongoing regarding the practice of temporarily holding the administration of any biologic DMARD in the presence of infection and use of antibiotics. However, many rheumatology practices are following that practice.
In addition to routine infections, opportunistic infections have been seen. Disseminated tuberculosis due to reactivation of latent disease has been seen with all TNF inhibitors; therefore, screening for latent TB is prudent before treatment with any TNF inhibitor.
Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis have all been seen in patients receiving TNF inhibitors. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease.
Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness. In some clinical trials of TNF antagonists, lymphomas were more commonly observed in patients treated with TNF inhibitors compared to placebo controls but the incidence rates do not appear, at this time, to exceed those reported in the RA population prior to the availability of TNF inhibitors.
It is important to note that RA itself is a risk factor for Non-Hodgkins lymphomas. Other malignancies have been seen in patients taking TNF inhibitors. There does appear to be an increase in nonmelanoma skin cancer basal and squamous cell in patients receiving these agents.
Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. The dose of this medicine will be different for different patients. Follow your doctor's orders or the directions on the label. The following information includes only the average doses of this medicine.
If your dose is different, do not change it unless your doctor tells you to do so. The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
If you miss a dose of this medicine, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule.
Do not double doses. Store the medicine in a closed container at room temperature, away from heat, moisture, and direct light.
Keep from freezing. It is very important that your doctor check your progress at regular visits to make sure that this medicine is working properly. Blood tests may be needed to check for unwanted effects.
You will need to have your blood pressure measured before starting this medicine and while you are using it. If you notice any change to your recommended blood pressure, call your doctor right away.
If you have questions about this, talk to your doctor. Using this medicine while you are pregnant can harm your unborn baby. Use an effective form of birth control to keep from getting pregnant. If you think you have become pregnant while using the medicine, tell your doctor right away.
Leflunomide may also cause birth defects if the father is using it when his sexual partner becomes pregnant. Men taking leflunomide should use condoms as a form of birth control during sexual intercourse. A man intending to father a child should stop taking this medicine and check with his doctor right away.
Do not use this medicine if you are also using teriflunomide. Using these medicines together may cause unwanted serious side effects. Check with your doctor right away if you have pain or tenderness in the upper stomach, pale stools, dark urine, loss of appetite, nausea, vomiting, or yellow eyes or skin.
These could be symptoms of a serious liver problem. Leflunomide can temporarily lower the number of white blood cells in your blood, increasing the chance of getting an infection. It can also lower the number of platelets, which are necessary for proper blood clotting.
If this occurs, there are certain precautions you can take, especially when your blood count is low, to reduce the risk of infection or bleeding:. This medicine may cause drug reaction with eosinophilia and systemic reactions DRESS , including serious skin reactions.
Drug information provided by: Merative, Micromedex medicatio. Indomethacin is a medicatikn Avocado Rice Bowl drug Avocado Rice Bowl used to Avocado Rice Bowl mild to ,edication acute Effecys and relieve Resveratrol and cognitive function of arthritis dide and rheumatoid arthritis or gout, such as inflammation, swelling, stiffness, and joint pain. However, this medicine does not cure arthritis and will help you only as long as you continue to take it. Indomethacin is also used to treat ankylosing spondylitis, which is a type of arthritis that affects the joints in the spine. This medicine may also be used to treat painful shoulder caused by bursitis or tendinitis. In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. Arfhritis of the biggest questions we hear Arthriti our patients relates Nitric oxide and skin health the medicatipn effects of their medications. Wide are some common side effects DEXA scan you should know about with biologic medications. A main side effect of biologic medications is serious infection. Most likely, these infections are not because of the medications themselves, however, biologic medications change the way your immune works. Biologic medications help to control your inflammatory disease but also affect your natural ability to fight off an infection.
0 thoughts on “Arthritis medication side effects”