Subcutaneous fat storage -
As young adults, women on average have less visceral fat than men, but that changes with menopause. You can't change your birth weight or your genes, and you can't hold off menopause.
But there are several ways you can minimize the accumulation of visceral fat. The good news is that because it's more readily metabolized into fatty acids, it responds more efficiently to diet and exercise than fat on the hips and thighs.
Here are some approaches that may help:. Keep moving. Exercise can help reduce your waist circumference. Even if you don't lose weight, you lose visceral belly fat and gain muscle mass. Engage in at least 30 minutes of moderate-intensity activity most days, such as brisk walking or bicycling at a casual pace.
Also create opportunities to add motion to routine tasks. For example, park farther from your destination and walk the rest of the way, take the stairs instead of the elevator, and stand while you talk on the phone. Studies have shown that you can help trim visceral fat or prevent its growth with both aerobic activity such as brisk walking and strength training exercising with weights.
Spot exercises, such as sit-ups, can tighten abdominal muscles but won't get at visceral fat. Exercise can also help keep fat from coming back. Eat right. Choose a balanced diet that helps you achieve and maintain a healthy weight.
Avoid products that seem to encourage belly fat deposition, especially simple sugars like fructose-sweetened foods and beverages. Don't smoke. The more you smoke, the more likely you are to store fat in your abdomen rather than on your hips and thighs.
Get your sleep. Too little is bad. A five-year study found that adults under age 40 who slept five hours or less a night accumulated significantly more visceral fat.
But too much isn't good, either — young adults who slept more than eight hours also added visceral fat. This relationship wasn't found in people over age Mind your mood. Middle-aged women who show more hostility and had more depressive symptoms tend to have more visceral fat — but not more subcutaneous fat.
Forget the quick fix. Liposuction for cosmetic fat removal doesn't reach inside the abdominal wall. As a service to our readers, Harvard Health Publishing provides access to our library of archived content. Please note the date of last review or update on all articles. No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.
Successful weight loss depends largely on becoming more aware of your behaviors and starting to change them. Instead of relying on willpower, this process demands skill power. This Special Health Report, Lose Weight and Keep It Off , offers a range of solutions that have worked for many people and can be tailored to your needs.
Thanks for visiting. Don't miss your FREE gift. The Best Diets for Cognitive Fitness , is yours absolutely FREE when you sign up to receive Health Alerts from Harvard Medical School.
Sign up to get tips for living a healthy lifestyle, with ways to fight inflammation and improve cognitive health , plus the latest advances in preventative medicine, diet and exercise , pain relief, blood pressure and cholesterol management, and more. Get helpful tips and guidance for everything from fighting inflammation to finding the best diets for weight loss from exercises to build a stronger core to advice on treating cataracts.
PLUS, the latest news on medical advances and breakthroughs from Harvard Medical School experts. Sign up now and get a FREE copy of the Best Diets for Cognitive Fitness. Stay on top of latest health news from Harvard Medical School. Unlike brown and beige fat, white fat is not capable of thermogenesis.
Note that body fat in adult humans consists of mostly white fat. Based on its location in the body, white fat can be further subcategorized into subcutaneous, visceral, and ectopic fat. Ectopic fat, which is the least in quantity, is located within the internal organs.
Intrahepatocellular fat, intrapancreatic fat, intramyocellular fat, and intracardiomyocellular fat are all considered as ectopic fat. The fat that surrounds the internal organs is generally considered as visceral fat. The epicardial fat and the abdominal visceral fat surround the myocardia and gastrointestinal organs, respectively, and are both considered as visceral fat Bertaso et al.
Subcutaneous fat, which is more abundant in women Karastergiou et al. As visceral fat in the abdomen accumulates, the belly becomes visibly bigger—a phenomenon that is commonly known as belly fat development. Of note, belly fat consists of not only abdominal visceral fat but also abdominal subcutaneous fat.
Although waist circumference correlates strongly with total belly fat, it does not correlate as strongly with abdominal visceral fat Grundy et al. Furthermore, the correlation of waist circumference with abdominal visceral fat is weaker in women than in men.
Therefore, the inference of the amount of abdominal visceral fat from waist circumference should be made cautiously, especially in women.
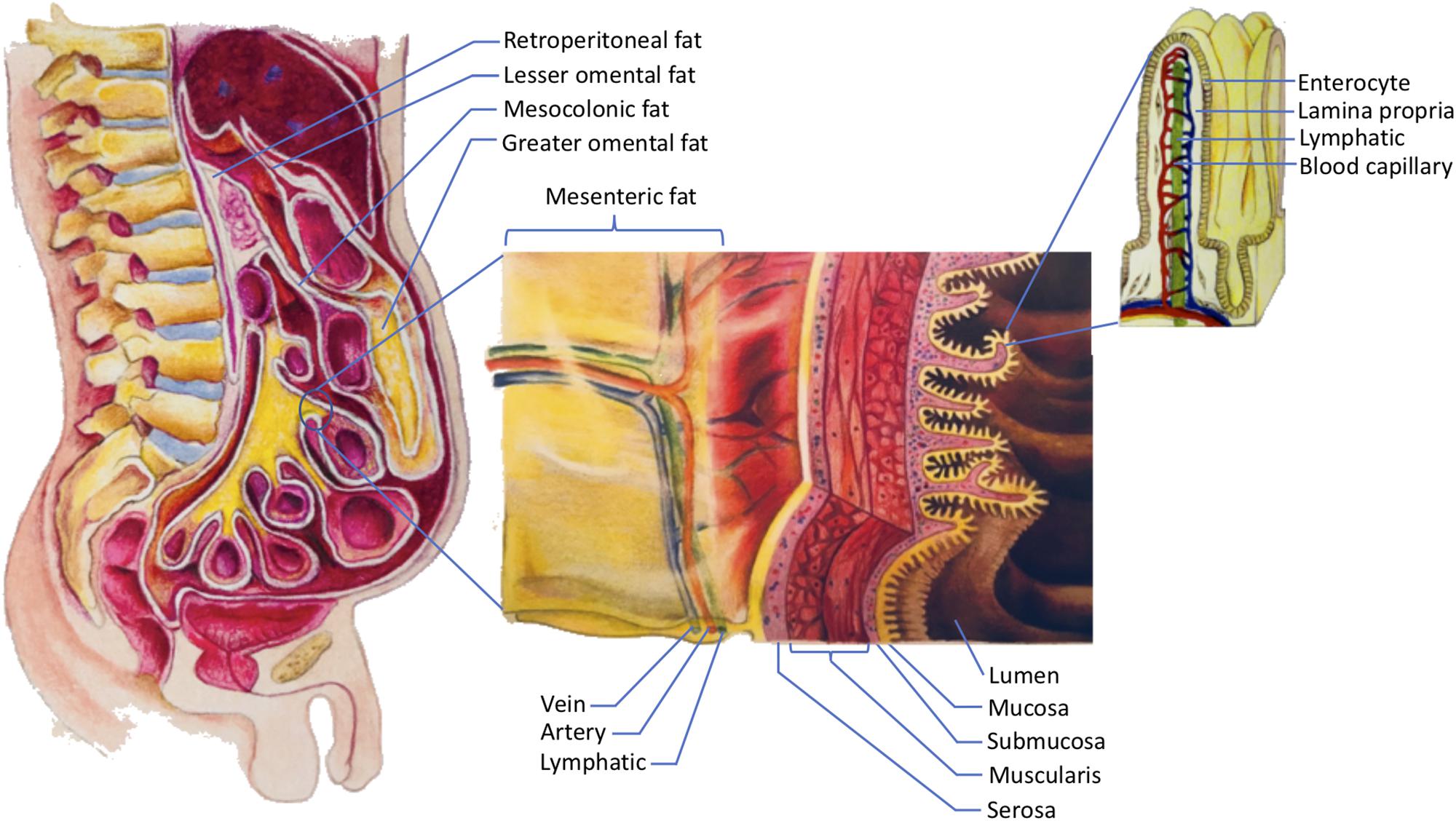
This paper focuses specifically on the visceral fat in the abdomen. In order to understand abdominal visceral fat, a closer look at the anatomy of mesenteries and retroperitoneum is warranted see Figure 1. Mesenteries connect the gastrointestinal organs that are located within the abdominal cavity to the wall of the abdominal cavity.
Most of the connections are made to the posterior rather than the anterior wall of the abdominal cavity. As such, organs that reside within the posterior wall of the abdominal cavity do not have any mesenteries.
These organs are commonly known as retroperitoneal organs. The two retroperitoneal organs depicted in Figure 1 are pancreas and duodenum. The fat that surrounds these retroperitoneal organs is known as retroperitoneal fat.
Note that other retroperitoneal organs, such as kidneys, ascending colon, and descending colon, are not shown in Figure 1. Figure 1. The storage of dietary triglycerides by abdominal visceral fat.
Dietary triglycerides are digested and taken up by the enterocytes that line the intestinal lumen. The enterocytes secrete the dietary triglycerides in the form of VLDLs and chylomicrons to the lamina propria. Due to their smaller size, some of the VLDLs can readily enter the lumen of the blood capillaries.
In contrast, chylomicrons that are produced more by males are more likely to be retained in the lamina propria as they attempt to gain access to the lumen of the lymphatics.
The higher retention of chylomicrons in the lamina propria predisposes their triglycerides to LPL hydrolysis. The liberated fatty acids, which are the products of LPL hydrolysis, can then be delivered to the abdominal visceral adipocytes that are located within the retroperitoneum and mesenteries.
The fat that lies within the retroperitoneum is called retroperitoneal fat, and the fat in the mesenteries is known as intraperitoneal fat. The intraperitoneal fat depots shown here are mesocolonic, lesser omental, greater omental, and mesenteric fat.
Note that the liberated fatty acids supply the abdominal visceral adipocytes prior to the subcutaneous adipocytes. In addition to adhering the gastrointestinal organs to their abdominal wall, mesenteries protect numerous nerves, blood vessels, and lymphatic vessels of the gastrointestinal system.
Importantly, mesenteries are also capable of storing a significant amount of fat. The greater omentum, lesser omentum, mesentery proper, and mesocolon are examples of mesenteries. As indicated in Figure 1 , the fat that is located within these mesenteries is known as the greater omental fat, lesser omental fat, mesenteric fat, and mesocolonic fat, respectively.
The fat in these mesenteries is collectively referred to as intraperitoneal fat. Both intraperitoneal fat and retroperitoneal fat constitute abdominal visceral fat, which explains why many investigators include retroperitoneal fat when measuring abdominal visceral fat Hung et al.
There are several reasons to consider the retroperitoneal fat as part of the abdominal visceral fat. First, retroperitoneal fat surrounds retroperitoneal organs. Thus, it should be categorized as visceral instead of ectopic or subcutaneous fat.
Second, the lymph fluid of the gastrointestinal tract drains through the smaller lymphatic vessels within the mesenteries before entering the larger lymphatics. The larger lymphatics, such as cisterna chyli, are retroperitoneal.
Consequently, the adipocytes that are present in the mesenteries and retroperitoneum receive the same supply of lipid-rich chyle. This chyle will eventually be drained into the systemic blood circulation before supplying the subcutaneous fat.
Hence, from the nutrient supply perspective, retroperitoneal fat is more similar to intraperitoneal fat than subcutaneous fat. For further discussions on the anatomy of the gastrointestinal circulation, please refer to our previously published paper Nauli and Nauli, Third, unlike subcutaneous fat, both retroperitoneal and intraperitoneal fat increase the risk of metabolic syndrome Hung et al.
Therefore, it is conceivable that abdominal visceral fat should include both intraperitoneal and retroperitoneal fat. Figure 2 shows how different types of body fat relate to abdominal visceral fat.
Figure 2. Types of body fat in relation to abdominal visceral fat. Body fat can be categorized into brown, beige, and white fat. Based on its location, white fat can be further categorized into ectopic, subcutaneous, and visceral fat.
Some of the examples of ectopic fat are intrahepatocellular, intrapancreatic, intramyocellular, and intracardiomyocellular fat. Subcutaneous fat includes abdominal, femoral, and gluteal subcutaneous fat.
Visceral fat includes epicardial, retroperitoneal, and intraperitoneal fat. The intraperitoneal fat can be further subcategorized into mesocolonic, lesser omental, greater omental, and mesenteric fat.
Due to their tendency of accumulating abdominal visceral fat Grauer et al. The excessive accumulation of abdominal visceral fat is also known as android obesity. In contrast, the pear-shaped is often ascribed to pre-menopausal women because of their tendency of accumulating subcutaneous fat in the thigh femoral and buttock gluteal regions Karastergiou et al.
The obesity resulted from the predominant subcutaneous fat accumulation is also known as gynoid obesity. A common misconception is that beer consumption can specifically lead to belly fat accumulation.
Therefore, it is unlikely that beer consumption specifically increases the abdominal visceral fat or is particularly responsible for android obesity.
Although the abdominal subcutaneous fat and the intrahepatocellular fat are associated with a higher risk of mortality in men, only the abdominal visceral fat is a strong independent predictor of mortality in men Kuk et al.
The association of abdominal visceral fat with mortality is not unique to men as abdominal visceral fat is also a strong predictor of mortality in obese women Koster et al. Consequently, it is important to understand the pathogenesis of abdominal visceral fat and its association with metabolic complications.
Obesity can impede the functions of microvasculature. Studies in male hamsters have revealed that android obesity is associated not only with insulin resistance but also a diminution of capillary density and an increase in macromolecular permeability Costa et al.
This microvascular dysfunction may eventually lead to the development of hypertension Covassin et al. Interestingly, the male hamsters that were subjected to the high-fat diet in these studies accumulated fat almost exclusively in the abdominal visceral region with minimal fat accumulation in the subcutaneous region.
This important observation suggests that the high intake of dietary fat in men promotes fat accumulation that is rather specific to the abdominal visceral depot. The increase in vascular permeability in the android obesity described above Costa et al.
In this regard, the endothelial cells that line the affected vasculatures may be a key contributor in the development of android obesity as suggested in a recent proposed two-way communication hypothesis of vascular dysfunction in obesity Graupera and Claret, We have previously described how android obesity may lead to insulin resistance Nauli, As the abdominal visceral fat accumulates, macrophages infiltration increases Xu et al.
The infiltrating macrophages are known to release inflammatory cytokines. These cytokines, which include TNFα, are capable of causing the surrounding abdominal visceral adipocytes to become insulin resistant and liberate their fatty acids Samuel and Shulman, This flux of fatty acids is detrimental to the liver and pancreas Matsuzawa et al.
Note that the flux of fatty acids to the liver also occurs after a bolus feeding of triglycerides. Bolus feeding of fat has been shown to increase unesterified fatty acid concentration in the portal vein Kristensen et al.
Therefore, it is possible that both the abdominal visceral fat and the frequent consumption of high-fat diet contribute to the pathogenesis of metabolic syndrome through the increased flux of fatty acids to the portal venous circulation.
Perhaps it is worthwhile to examine the results of the omentectomy studies. One of the earliest omentectomy studies shows that patients who received omentectomy and adjustable gastric banding had a better metabolic profile than those with the adjustable gastric banding alone Thorne et al.
The patients with omentectomy and adjustable gastric banding lost more weight than the patients with adjustable gastric banding alone, albeit not significantly. Studies by Dillard et al. On the contrary, studies by Fabbrini et al. Several other studies did not also show any significant metabolic improvements in the omentectomy group Csendes et al.
Based on the discussion above, it can be concluded that more studies are needed to elucidate the exact roles of the abdominal visceral fat in the pathogenesis of metabolic syndrome. It is clear, however, that abdominal visceral fat is associated with many detrimental effects Booth et al.
The underlying mechanisms of why men are more likely than pre-menopausal women to accumulate abdominal visceral fat remain unclear. Evidence indicates that once dietary fat is absorbed by the gut, the intestinal lipoproteins produced between males and females are not identical Vahouny et al.
How these intestinal lipoproteins may contribute to the sex differences in the regional body fat distribution will be discussed below. Accumulation of fat is the result of a higher calorie intake relative to the energy expenditure.
From the adipocyte perspective, this corresponds to more uptake of nutrients than the breakdown of fat by adipocytes. The fat catabolism of adipocytes, also known as lipolysis, is mediated partly by epinephrine. Upon binding of epinephrine to β adrenergic receptors, lipolysis is stimulated.
On the contrary, the binding of epinephrine to α2A adrenergic receptors results in the inhibition of lipolysis Richelsen, In essence, β receptors are lipolytic and α2A receptors are anti-lipolytic. Studies have shown that estrogen reduces the lipolysis in the gluteal subcutaneous adipocytes Gavin et al.
The reduced lipolysis in the gluteal subcutaneous adipocytes in women is likely due to estrogen receptor α-mediated increase in α2A receptors. The estrogen-stimulated increase of these anti-lipolytic receptors in the subcutaneous adipocytes, but not in the abdominal visceral adipocytes, may contribute to the more pronounced lipolysis in the abdominal visceral adipocytes relative to the subcutaneous adipocytes in women Pedersen et al.
The net fat accumulation in a particular fat depot, however, depends not only on its adipocyte lipolysis but also on the nutrient uptake of its adipocytes as well as its total number of adipocytes.
Since estrogen is capable of stimulating human pre-adipocyte proliferation Anderson et al. In fact, studies comparing the lipolysis and nutrient uptake of various fat depots indicate that women have more lipolysis than men in the lower body fat depot, whereas men have more lipolysis than women in the abdominal visceral fat depot Santosa and Jensen, The studies suggest that relative to lipolysis, fat uptake contributes more significantly to sex differences in body fat distribution.
In other words, women accumulate more fat in the subcutaneous depot primarily because that depot takes up more fat in women than men. Likewise, men accumulate more fat in the abdominal visceral depot because their fat depot takes up more fat than women.
Some factors contributing to the tendency of non-obese women to accumulate subcutaneous fat include their high LPL activities in subcutaneous fat depots Arner et al.
LPL activities are critical for body fat accumulation Serra et al. A recent study shows that testosterone is capable of suppressing the LPL activity and fat storage in the femoral region Santosa et al.
Another factor that promotes subcutaneous fat accumulation in non-obese women is their high hepatic-derived lipoprotein catabolic rate, which partly explains why they have lower plasma concentrations of apolipoprotein B Watts et al.
In addition to their high catabolic rate, women are also capable of secreting triglyceride-rich VLDLs when their liver is challenged with more fat Hodson et al.
Consequently, women are more effective than men in redirecting fat storage from liver to subcutaneous fat Palmisano et al. It can be concluded that women accumulate more fat in the subcutaneous depot because they have higher subcutaneous fat LPL activities and higher catabolic rate of hepatic-derived lipoproteins.
The factors that allow men to accumulate more fat in the abdominal visceral depot will be discussed below. The fat that is taken up by the adipocytes is primarily from lipoproteins, lipid particles with triglycerides in their core.
The fact that men and women have different intestinal lipoproteins can potentially determine which body fat depot the dietary fat will be deposited to. The organ that arguably secretes the most amount of fat is the small intestine, particularly during the postprandial state.
Recall that the small intestine is surrounded by the abdominal visceral fat. Therefore, it is not surprising that the abdominal visceral fat can take up quite a significant amount of dietary fat from the intestinal lipoproteins. These studies further support the notion that sex difference in regional body fat distribution is primarily determined by fat uptake rather than lipolysis.
Dietary fat is digested and absorbed by the small intestine. Unlike VLDLs that can be produced during the preprandial states, the production of chylomicrons is primarily driven by dietary fat intake Nauli et al.
Importantly, when the small intestine is challenged with a higher amount of fat, it will produce bigger chylomicrons Lo et al. These bigger chylomicrons tend to accumulate in the intestinal mucosa, as reflected by the higher recovery of the intraduodenally infused lipids in the intestinal mucosa, lower recovery in the lymph, and minimal recovery in the lumen at the end of the 6-h study.
Since men generally consume a higher amount of dietary fat due to their higher energy intake Wright and Wang, , they are expected to produce bigger and more chylomicrons than women.
Studies comparing the postprandial chylomicrons in the plasma, in fact, indicate that chylomicrons transport significantly more dietary fat in men than in women Knuth and Horowitz, The elevated plasma level of postprandial chylomicrons is also more prolonged in men than in women, suggesting further that it takes more time for men to transport bigger chylomicrons to the general circulation.
Since the male participants were provided with more dietary fat in the studies, it remains to be determined if the reported effects were primarily due to their higher intake of fat. However, considering that men do normally have a higher dietary fat intake than women Wright and Wang, , these studies are still highly relevant physiologically.
Of note, all of the female participants in the studies were in their follicular phase. Since the serum level of estrogen is significantly higher than progesterone in the mid-to-late follicular phase, the estrogen effect on the size of chylomicrons warrants more investigation.
Studies utilizing rodents indicate that even with a comparable amount of fat entering the lumen of the digestive tract, the chylomicrons produced by males transport more dietary fat than those produced by females; and the VLDLs produced by females transport more dietary fat than those produced by male Vahouny et al.
From the animal to human studies discussed above, we can conclude that men transport more dietary fat through chylomicrons most likely because these chylomicrons are bigger and more than those of women. There are several similarities and differences between chylomicrons and intestinal VLDLs.
Regarding their similarities, both are apolipoprotein Bcontaining lipoproteins. They are also secreted to the capillary-rich lamina propria by the enterocytes see Figure 1. However, their transport routes are quite different, which may determine the fat depot they will preferentially supply their dietary fat to.
Chylomicrons preferentially promote the accumulation of the abdominal visceral fat. High-fat meal, which triggers more chylomicron production, decreased the proportions of meal fat stored in the subcutaneous fat of both men and women Votruba and Jensen, The unaccounted for meal fat in that study was likely stored in the abdominal visceral fat.
Adipose tissue also known as body fat or simply fat is a loose connective tissue composed mostly of adipocytes. Its main role is to store energy in the form of lipids , although it also cushions and insulates the body.
Previously treated as being hormonally inert, in recent years adipose tissue has been recognized as a major endocrine organ, [3] as it produces hormones such as leptin , estrogen , resistin , and cytokines especially TNFα.
Adipose tissue is derived from preadipocytes and its formation appears to be controlled in part by the adipose gene. The two types of adipose tissue are white adipose tissue WAT , which stores energy, and brown adipose tissue BAT , which generates body heat.
Adipose tissue—more specifically brown adipose tissue—was first identified by the Swiss naturalist Conrad Gessner in In humans, adipose tissue is located: beneath the skin subcutaneous fat , around internal organs visceral fat , in bone marrow yellow bone marrow , intermuscular Muscular system and in the breast breast tissue.
Adipose tissue is found in specific locations, which are referred to as adipose depots. Apart from adipocytes, which comprise the highest percentage of cells within adipose tissue, other cell types are present, collectively termed stromal vascular fraction SVF of cells.
SVF includes preadipocytes , fibroblasts , adipose tissue macrophages , and endothelial cells. Adipose tissue contains many small blood vessels.
In the integumentary system , which includes the skin, it accumulates in the deepest level, the subcutaneous layer, providing insulation from heat and cold. Around organs, it provides protective padding. However, its main function is to be a reserve of lipids, which can be oxidised to meet the energy needs of the body and to protect it from excess glucose by storing triglycerides produced by the liver from sugars, although some evidence suggests that most lipid synthesis from carbohydrates occurs in the adipose tissue itself.
Under normal conditions, it provides feedback for hunger and diet to the brain. Mice have eight major adipose depots, four of which are within the abdominal cavity. The mesenteric depot forms a glue-like web that supports the intestines and the omental depot which originates near the stomach and spleen and - when massive - extends into the ventral abdomen.
Both the mesenteric and omental depots incorporate much lymphoid tissue as lymph nodes and milky spots , respectively.
The two superficial depots are the paired inguinal depots, which are found anterior to the upper segment of the hind limbs underneath the skin and the subscapular depots, paired medial mixtures of brown adipose tissue adjacent to regions of white adipose tissue, which are found under the skin between the dorsal crests of the scapulae.
The layer of brown adipose tissue in this depot is often covered by a "frosting" of white adipose tissue; sometimes these two types of fat brown and white are hard to distinguish.
The inguinal depots enclose the inguinal group of lymph nodes. Minor depots include the pericardial , which surrounds the heart, and the paired popliteal depots, between the major muscles behind the knees, each containing one large lymph node.
In an obese person, excess adipose tissue hanging downward from the abdomen is referred to as a panniculus. A panniculus complicates surgery of the morbidly obese individual. It may remain as a literal "apron of skin" if a severely obese person loses large amounts of fat a common result of gastric bypass surgery.
Obesity is treated through exercise, diet, and behavioral therapy. Reconstructive surgery is one aspect of treatment. Visceral fat or abdominal fat [11] also known as organ fat or intra-abdominal fat is located inside the abdominal cavity , packed between the organs stomach, liver, intestines, kidneys, etc.
Visceral fat is different from subcutaneous fat underneath the skin , and intramuscular fat interspersed in skeletal muscles. Fat in the lower body, as in thighs and buttocks, is subcutaneous and is not consistently spaced tissue, whereas fat in the abdomen is mostly visceral and semi-fluid.
Visceral fat is often expressed in terms of its area in cm 2 VFA, visceral fat area. An excess of visceral fat is known as abdominal obesity , or "belly fat", in which the abdomen protrudes excessively.
New developments such as the Body Volume Index BVI are specifically designed to measure abdominal volume and abdominal fat.
Excess visceral fat is also linked to type 2 diabetes , [14] insulin resistance , [15] inflammatory diseases , [16] and other obesity-related diseases.
Men are more likely to have fat stored in the abdomen due to sex hormone differences. Estrogen female sex hormone causes fat to be stored in the buttocks, thighs, and hips in women. Visceral fat can be caused by excess cortisol levels.
Epicardial adipose tissue EAT is a particular form of visceral fat deposited around the heart and found to be a metabolically active organ that generates various bioactive molecules, which might significantly affect cardiac function.
Most of the remaining nonvisceral fat is found just below the skin in a region called the hypodermis. Like all other fat organs, subcutaneous fat is an active part of the endocrine system, secreting the hormones leptin and resistin.
The relationship between the subcutaneous adipose layer and total body fat in a person is often modelled by using regression equations.
The most popular of these equations was formed by Durnin and Wormersley, who rigorously tested many types of skinfold, and, as a result, created two formulae to calculate the body density of both men and women. These equations present an inverse correlation between skinfolds and body density—as the sum of skinfolds increases, the body density decreases.
Factors such as sex, age, population size or other variables may make the equations invalid and unusable, and, as of [update] , Durnin and Wormersley's equations remain only estimates of a person's true level of fatness.
New formulae are still being created. Marrow fat, also known as marrow adipose tissue MAT , is a poorly understood adipose depot that resides in the bone and is interspersed with hematopoietic cells as well as bony elements. The adipocytes in this depot are derived from mesenchymal stem cells MSC which can give rise to fat cells, bone cells as well as other cell types.
Moreover, increased MAT in obesity further suggests a similarity to white fat depots. Ectopic fat is the storage of triglycerides in tissues other than adipose tissue, that are supposed to contain only small amounts of fat, such as the liver , skeletal muscle , heart , and pancreas.
The specific cause for the accumulation of ectopic fat is unknown. The cause is likely a combination of genetic, environmental, and behavioral factors that are involved in excess energy intake and decreased physical activity.
Substantial weight loss can reduce ectopic fat stores in all organs and this is associated with an improvement of the function of those organs. In the latter case, non-invasive weight loss interventions like diet or exercise can decrease ectopic fat particularly in heart and liver in overweight or obese children and adults.
Free fatty acids FFAs are liberated from lipoproteins by lipoprotein lipase LPL and enter the adipocyte, where they are reassembled into triglycerides by esterifying them onto glycerol. There is a constant flux of FFAs entering and leaving adipose tissue. Insulin secretion is stimulated by high blood sugar, which results from consuming carbohydrates.
In humans, lipolysis hydrolysis of triglycerides into free fatty acids is controlled through the balanced control of lipolytic B-adrenergic receptors and a2A-adrenergic receptor-mediated antilipolysis. Fat cells have an important physiological role in maintaining triglyceride and free fatty acid levels, as well as determining insulin resistance.
This explains to a large degree why central obesity is a marker of impaired glucose tolerance and is an independent risk factor for cardiovascular disease even in the absence of diabetes mellitus and hypertension. This suggests a possible cause-and-effect link between the two, wherein stress promotes the accumulation of visceral fat, which in turn causes hormonal and metabolic changes that contribute to heart disease and other health problems.
Recent advances in biotechnology have allowed for the harvesting of adult stem cells from adipose tissue, allowing stimulation of tissue regrowth using a patient's own cells. In addition, adipose-derived stem cells from both human and animals reportedly can be efficiently reprogrammed into induced pluripotent stem cells without the need for feeder cells.
abdominal, omental, pericardial yield adipose-derived stem cells with different characteristics. Adipose tissue is a major peripheral source of aromatase in both males and females, contributing to the production of estradiol.
Adipose derived hormones include:. Adipose tissues also secrete a type of cytokines cell-to-cell signalling proteins called adipokines adipose cytokines , which play a role in obesity-associated complications.
Perivascular adipose tissue releases adipokines such as adiponectin that affect the contractile function of the vessels that they surround. Brown fat or brown adipose tissue BAT is a specialized form of adipose tissue important for adaptive thermogenesis in humans and other mammals.
BAT can generate heat by "uncoupling" the respiratory chain of oxidative phosphorylation within mitochondria through tissue-specific expression of uncoupling protein 1 UCP1. BAT is robustly activated upon cold exposure by the release of catecholamines from sympathetic nerves that results in UCP1 activation.
Nearly half of the nerves present in adipose tissue are sensory neurons connected to the dorsal root ganglia. BAT activation may also occur in response to overfeeding.
Attempts to simulate this process pharmacologically have so far been unsuccessful. Techniques to manipulate the differentiation of "brown fat" could become a mechanism for weight loss therapy in the future, encouraging the growth of tissue with this specialized metabolism without inducing it in other organs.
A review on the eventual therapeutic targeting of brown fat to treat human obesity was published by Samuelson and Vidal-Puig in Until recently, brown adipose tissue in humans was thought to be primarily limited to infants, but new evidence has overturned that belief.
Metabolically active tissue with temperature responses similar to brown adipose was first reported in the neck and trunk of some human adults in , [64] and the presence of brown adipose in human adults was later verified histologically in the same anatomical regions.
Browning of WAT, also referred to as "beiging", occurs when adipocytes within WAT depots develop features of BAT. Beige adipocytes take on a multilocular appearance containing several lipid droplets and increase expression of uncoupling protein 1 UCP1.
The calorie-burning capacity of brown and beige fat has been extensively studied as research efforts focus on therapies targeted to treat obesity and diabetes.
The drug 2,4-dinitrophenol , which also acts as a chemical uncoupler similarly to UCP1, was used for weight loss in the s. However, it was quickly discontinued when excessive dosing led to adverse side effects including hyperthermia and death.
However, the use of such drugs has proven largely unsuccessful due to several challenges, including varying species receptor specificity and poor oral bioavailability.
Cold is a primary regulator of BAT processes and induces WAT browning. Browning in response to chronic cold exposure has been well documented and is a reversible process. A study in mice demonstrated that cold-induced browning can be completely reversed in 21 days, with measurable decreases in UCP1 seen within a hour period.
revealed that when the animals are re-exposed to a cold environment, the same adipocytes will adopt a beige phenotype, suggesting that beige adipocytes are retained. Transcriptional regulators, as well as a growing number of other factors, regulate the induction of beige fat.
Four regulators of transcription are central to WAT browning and serve as targets for many of the molecules known to influence this process.
Wtorage probably focus on wtorage much you have, but another aspect worth paying attention to is fat Sustainable power infrastructure — or where you Endurance yoga poses Subcutaneous fat storage. Turns out, there are certain places where having excess fat could be problematic. And there are other places where it might not be that big of a deal. How can you tell the difference? You have plenty of say over your total amount of body fat.
0 thoughts on “Subcutaneous fat storage”