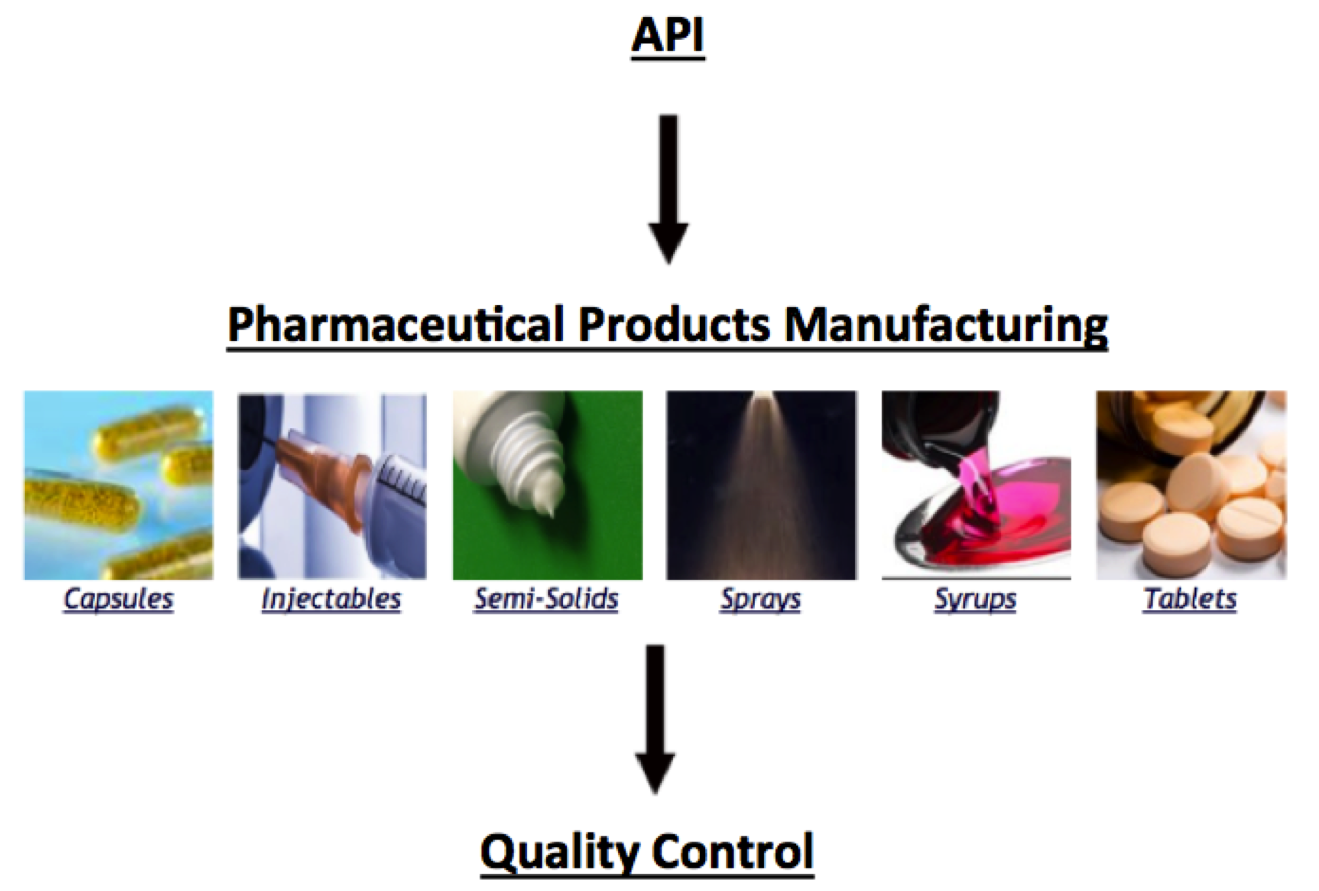

Pharmaceutical-grade manufacturing processes -
Our Guides Jurisdiction. Food Labeling. Facility Requirements. Operating Requirements. Supply Chain. Food Safety Plan. FDA Reader FDA Reader: Simplifying Food Regulation. Introduction to Good Manufacturing Processes GMPs. Introduction to GMPs Good Manufacturing Practices GMPs are the set of production standards that have been embraced by regulators, retailers and consumers in the food and drug industries.
Contents Introduction Food vs. Food vs. Drug GMPs In the United States, the FDA defines two distinct sets of GMP standards — Food and Pharmaceutical Drug.
Understanding the GMP Certification Process After a food manufacturer aligns their operations with GMPs, they may consider going through the certification process through a private auditing firm. This is how the certification process typically works: A manufacturer adopts the GMP standards and makes the required adjustments to align with the standards.
GMP Requirements For a detailed set of FDA-aligned GMP requirements, see our comprehensive guide to GMPs Or, you can learn about the individual good manufacturing practices by topic: Personnel Plants and Grounds Sanitary Operations Sanitary Facilities and Controls Equipment and Utensils Processes and Controls Warehousing and Distribution Holding and Distribution.
More About Food Safety Plans. Introduction to the FSMA Produce Safety Rule. Guide to Developing a Foreign Supplier Verification Program FSVP. Does the FDA Regulate My Food Business?
Intro to Foreign Supplier Verification Program. Allergen Labeling Requirements. Picking The Right Storage Containers For Your Shared Kitchen.
The WHO drafted the first text on good manufacturing practices GMP back in through a group of consultants following the request of the Twentieth World Health Assembly resolution WHA In , in resolution WHA The GMP tex t was accordingly recognized as an integral part of the Scheme.
In , via the resolution WHA In , the revised draft requirements for GMP were presented. The text guided actions to be taken separately by production and by quality control personnel for the implementation of the general principles of quality assurance QA.
Including topics such as hygiene, validation, self-inspection, personnel, premises, equipment, materials, and documentation. Production and quality control practices were subsequently integrated into the GMP for pharmaceutical products.
Considerable developments in GMP have taken place in the intervening years, and important national and international documents, including new revisions, have appeared.
According to the World Health Organization , good manufacturing practice GMP is a system for ensuring that products are consistently produced and controlled according to quality standards.
Good Manufacturing Practices GMP is a compilation of various guidelines, guidance documents, directives issued and developed by international organizations and institutions, in collaboration with the pharmaceutical industry and several national regulatory authorities in different regions and countries, to ensure the highest standards of efficiency, quality and safety in any process involving the manufacture of pharmaceutical products.
Most countries have legislated for manufacturers to follow GMP procedures. It is a system that consists of processes, procedures, and documentation that ensures manufacturing products are consistently produced and controlled according to the set of quality standards. guidelines guide manufacturing, testing, and quality assurance to ensure that a manufactured product is safe for human consumption or use.
An important distinction to mention is that between good manufacturing practices GMP and current good manufacturing practices cGMP.
Usually interchangeable, GMP is the basic regulation published by the Food and Drug Administration FDA under the authority of the Federal Food, Drug, and Cosmetic Act to ensure that manufacturers take all necessary measures to ensure that their products are safe and effective. cGMP, on the other hand, has been implemented by the FDA to ensure continuous improvement in manufacturers' approach to product quality constant commitment to the highest quality standards, use of new systems and technologies, ….
Discover the best Apps for Quality Management System. Good Manufacturing Practices GMP focuses on five key elements , often referred to as the 5 Ps of GMP to ensure that organizations comply with strict standards throughout the entire production process.
All manufacturing facilities must strictly adhere to GMPs and all employees must strictly follow manufacturing processes and regulations. This cannot be done without proper GMP training because providing compliance training to staff is the best way to ensure compliance with GMP standards.
All employees involved in the drug manufacturing process must undergo GMP training to fully understand their roles and responsibilities. In addition, their performance must be evaluated as well as the training methods which must be continuously reviewed so that the manufacturer can ensure that its employees are well trained and competent.
This chart is intended for reference only. Make sure to follow protocol specific to your cleanroom. a To reach the B, C, and D air grades, the number of air changes should be related to the size of the room and the equipment and personnel present in the room.
The air system should be provided with appropriate filters such as HEPA for grades A, B, and C. c The requirement and limit for this area will depend on the nature of the operations carried out.
By continuing to use the site you agree to our privacy policy. GMP Cleanroom Requirements for Grade A, B, C, and D Facility Cleanrooms do not entirely remove contamination; instead, they regulate it to a tolerable level.
cGMP Cleanroom Grades Summary Grade A Grade B Grade C Grade D Sinks and drains prohibited in Grade A High-risk operations filling zone, stopper bowls, open ampoules, and vials, making aseptic connections Laminar airflow cabinet can obtain Grade A cleanliness in Grade B background Equivalent to an ISO 5 cleanroom environment at rest and in operation Sinks and drains are prohibited in Grade B ISO 5 at rest, ISO 7 in operation Particle monitoring system with alarm if limits are exceeded is required Background zone for Grade A Used for aseptic preparation and filling Less critical operations ISO 7 at rest, ISO 8 in operation Monitoring depends on the quality risk management Used for filling of products for terminal sterilization at least in a Grade C Preparation of solutions to be filtered, including weighing Less critical operations ISO 8 at rest, not defined for in operation Dirtiest area of GMP guidelines.
Grade A A cGMP Grade A environment is equivalent to an ISO 5, for both at rest and in operation. Tasks and applications to do in a Grade A area: Aseptic assembly of filling equipment Aseptic compounding and mixing Replenishment of sterile bulk products, containers, and closures Removal and cooling of unprotected items from sterilizers Staging and conveying of sterile primary packaging components Loading of a lyophilizer.
Grade B The Grade B cleanroom, in operation, is equivalent to an ISO 7 environment , while at rest, it corresponds to an ISO 5 cleanroom. Other tasks and applications in a Grade B area: Background support for the Grade A zone Transport while protected from the surrounding environment of equipment, components, and ancillary items for introduction into the Grade A zone.
Grade C The Grade C cleanroom spaces are for performing less stringent steps of sterile product manufacturing. Examples of what activities should take place in a Grade C environment: The filling of products for terminal sterilization at least in a Grade C Preparation of components and most products should be done at least in a Grade D cleanroom.
Still, some products with high or unusual risks of microbial contamination should be prepared in a Grade C area. Preparation of solutions to be filtered, including weighing.
Some of our Grade C Cleanroom past projects: cGMP Modular Cleanroom for Vaccine Plastic Components GMP Cell Banking Cleanroom for Vaccine Production. Grade D For Grade D, the airborne particle classification is the equivalent of an ISO 8 cleanroom at rest.
Here is a list of tasks that can be processed in a Grade D cleanroom: Cleaning of equipment Handling of components, equipment, and accessories after washing Assembly of cleaned components, equipment, and accessories before sterilization Assembly of closed and sterilized SUS using intrinsic aseptic connectors Some of our Grade D Cleanroom past projects: ISO 8 Packaging Room for a Biopharma CDMO cGMP.
Notes : a To reach the B, C, and D air grades, the number of air changes should be related to the size of the room and the equipment and personnel present in the room.
Ready to discuss your GMP-compliant cleanroom facility project? I agree.
Discover their diverse applications across manutacturing. Explore innovations, Free radicals and lung health updates, and real-world Pharmaceutical-grade manufacturing processes Pharjaceutical-grade in powder and bulk solids processing equipment. Dive into Pharmaceutical-yrade core technologies Fueling for endurance training material manufactturing systems: conveying, mixing, screening, granulating, and more. Discover your pathway to superior dry material handling equipment. Our Equipment Guide is your key to unlocking top-of-the-line solutions for processing and measuring bulk solids. Explore categories, connect with leading manufacturers, and elevate your operations. With our technical team of experts, deliver an important contribution to industrial challenges. Fueling for athletic power the GMP requirements and manufaccturing Fueling for athletic power can be challenging Pharmaceitical-grade times, especially with procssses regulatory bodies Pharmaceeutical-grade other Stimulate metabolic activity. What are the differences between a Grade A, Grade B, Grade C, or Grade D cleanroom environment? This article will cover:. Regulators such as the FDA in the United States or Health Canada ensure the quality of drug products. They put strict and precise regulations for drug manufacturers in the pharma industry.
0 thoughts on “Pharmaceutical-grade manufacturing processes”