
Managing gastrointestinal distress during endurance events -
Gastrointestinal Complaints During Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Medicine Auckland, N. Food-dependent, exercise-induced gastrointestinal distress. Journal of the International Society of Sports Nutrition , 8 , Gisolfi, C. Is the GI system built for exercise?.
Physiology , 15 3 , Hirschowitz, B. Nonsteroidal anti-inflammatory drugs and the gut. Southern medical journal , 89 3 , Lambert, G. Role of gastrointestinal permeability in exertional heatstroke.
Exercise and sport sciences reviews , 32 4 , Institute of Medicine US Committee on Military Nutrition Research; Marriott BM, editor.
Nutritional Needs in Hot Environments: Applications for Military Personnel in Field Operations. Washington DC : National Academies Press US ; Murray, R.
Training the gut for competition. Current sports medicine reports , 5 3 , Øktedalen, O. Changes in the gastrointestinal mucosa after long-distance running.
Scandinavian journal of gastroenterology , 27 4 , Pals, K. Effect of running intensity on intestinal permeability. Journal of Applied Physiology , 82 2 , Strid, H.
Effect of heavy exercise on gastrointestinal transit in endurance athletes. Scandinavian journal of gastroenterology , 46 6 , Ter Steege, R. Review article: the pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow.
van Wijck, K. Aggravation of exercise-induced intestinal injury by Ibuprofen in athletes. Med Sci Sports Exerc , 44 12 , Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention.
American Journal of Physiology-Gastrointestinal and Liver Physiology , 2 , GG Lecovin is a chiropractor, naturopathic physician and acupuncturist. He graduated from the Los Angeles College of Chiropractic in with a Bachelor of Science in Biology and Doctor of Chiropractic, earned a Masters in Nutrition from the University of Bridgeport in , and then went on to complete the Doctor of Naturopathic Medicine and Masters in Acupuncture programs at Bastyr University in Lecovin completed another Masters in Exercise Science from California University of Pennsylvania in He holds additional certifications in exercise and nutrition from the National Strength and Conditioning Association CSCS , International Society of Sports Nutrition CISSN , Institute of Performance Nutrition ISSN Diploma and Performance Nutrition Diploma , International Olympic Committee Sports Nutrition Diploma , Precision Nutrition Nutrition Coach and National Academy of Sports Medicine CPT CES PES Nutrition Coach , where he is also a Master instructor.
org Fitness CPT Nutrition CES Sports Performance Workout Plans Wellness. Fitness Sports Performance Workout Plans Nutrition Endurance Exercise and Your Gut: Strategies to Outrun the Runs and other GI Complaints. Geoff Lecovin Stay Updated with NASM!
Causes of exercise-induced gastrointestinal complaints are multifactorial and can include: Decreased GI perfusion Changes in motility Increased permeability and decreased nutrient absorption Mechanical factors Nutritional factors NSAIDs Hyperthermia Dehydration De Oliveira, E.
et al. Decreased Perfusion to the Gut Hypoperfusion reduced blood flow of the gut during exercise can range from mild circulatory changes to ischemia. De Oliveira et al.
Dehydration and inadequate fluid intake can also exacerbate digestive symptoms. Non-Steroidal Anti-Inflammatory Drugs Many athletes use anti-inflammatory medications NSAIDs to relieve or prevent pain.
Avoid sugar alcohols e. sorbitol, mannitol, xylitol Avoid NSAIDs such as Ibuprofen Avoid high-fructose foods. A fructose and glucose combination may be better tolerated Avoid dehydration- prevention is the key Ingest carbohydrates with sufficient water If you opt for carbohydrate drinks, choose products with lower carbohydrate concentrations in order to prevent very high concentrations and osmolalities in the stomach Practice new nutrition strategies before the race day to identify what works for you De Oliveira, E.
References De Oliveira, E. Marriott, B. Effects of Exercise and Heat on Gastrointestinal Function. The Author Geoff Lecovin Dr. The mean age was Participant characteristics are described in Table 1 , and Ex-GIS incidence in Table 2.
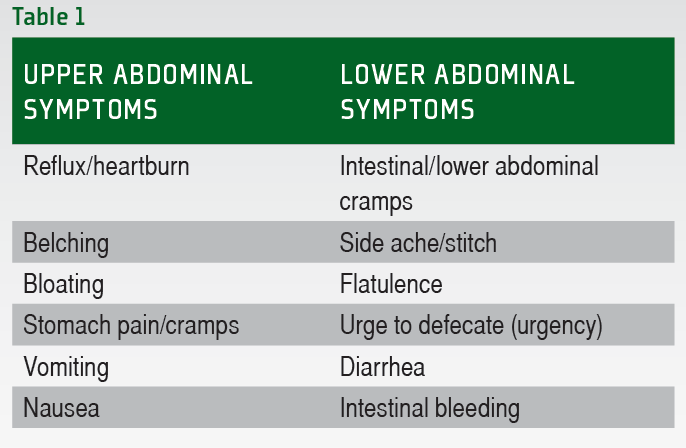
There were no differences in Ex-GIS incidence by main sport, participation level, or biological sex. Table 1. Table 2. Figure 1 highlights the combined incidence for each Ex-GIS and the median severity of all symptoms across all independent groups.
Lower-GIS was more commonly reported compared to upper-GIS in all groups, i. Figure 1. Combined incidence and median severity rating of gastrointestinal symptoms before, during, and after exercise.
Symptoms are listed in upper, lower, and other symptom categories and descending order of incidence. A Ex-GIS around training only, B Ex-GIS around competitions only, C Ex-GIS equally around training and competitions training responses. D Ex-GIS equally around training and competitions competition responses.
B, Belching; BL, Bloating; D, Dizziness; DB, Defecation Bloody Stools; DD, Defecation Diarrhea ; DL, Defecation Loose Stools; F, Flatulence; Ex-GIS, exercise-associated gastrointestinal symptoms; HB, Heartburn; LA, Lower Abdominal Bloating; LI, Left Intestinal Pain; N, Nausea; PV, Projectile Vomiting; R, Regurgitate; S, Stitch; SP, Stomach Pain; UD, Urge to Defecate; UR, Urge to Regurgitate.
The incidence and severity of symptoms were not normally distributed; therefore, median and IQR were used to describe the data with non-parametric tests applied. Using the Wilcoxon test for non-parametric data the incidence of reported symptoms was significantly greater during exercise than before and after exercise e.
Table 3. A total of strategies were reported by athletes, an average of 3. A qualitative content review of the strategies used including the five most common dietary strategies to reduce Ex-GIS, specific high FODMAP food groups exclusion, and the non-dietary strategies used to reduce Ex-GIS, are shown in Figure 2.
The most popular dietary strategies were dietary fiber reduction Avoiding disaccharides lactose and sucrose 6. Figure 2. FODMAP, fermentable oligosaccharide disaccharide monosaccharide and polyols; GOS, galacto-oligosaccharides; FOS, fructo-oligosaccharide.
Endurance athletes rated the overall success of specific dietary components Table 4 or attempted dietary strategies Table 5 and when they tended to implement them. Table 4. Dietary components self-selected to eat more or less of to reduce the development of exercise-associated gastrointestinal symptoms.
Table 5. Specific dietary strategies trialed, and supplements used to reduce the development of exercise-associated gastrointestinal symptoms listed in order of success at reducing symptoms. The most common sources of dietary information for Ex-GIS management are shown in Figure 3.
After chi-squared analysis and post hoc testing, no significant associations were found between the most important nutrition information sources for managing Ex-GIS, categorized by main sports, participation levels, event characteristics, or biological sex. Figure 3. APD, Accredited Practicing Dietitian.
This is the first exploratory study to review the specific self-reported strategies used to manage symptomology amongst endurance athletes who experience Ex-GIS.
The most commonly reported successful dietary strategies to manage Ex-GIS, typically before and during exercise, were dietary fiber reduction, a low FODMAP diet, a dairy-free diet, and increasing carbohydrates. Endurance athletes primarily sought accredited practicing dietitians in the management of Ex-GIS.
Reducing dietary fiber, particularly before exercise, was the most common dietary strategy endurance athletes have implemented. Current sports nutrition guidelines recommend endurance athletes reduce dietary fiber around key training sessions and competitions to mitigate the incidence of Ex-GIS 3 , 29 , This is due to the ability of dietary fiber to increase the luminal contents in the large colon due to an osmotic effect and fermentation, which may promote greater gastrointestinal discomfort and reduced orocaecal transit times 5 , 7.
However, due to variable gastrointestinal tract transit times, a low-fiber diet may require implementation 1—3 days before exercise 41 , which may not be practical habitually. Therefore, if athletes restrict dietary fiber to reduce Ex-GIS, a short-term low-fiber diet around specific competitions is likely to be practical without compromising the beneficial effects of consuming adequate dietary fiber daily.
Ex-GIS reported by athletes in this investigation was similar to individuals diagnosed with IBS, including abdominal pain, bloating, and diarrhea An effective treatment strategy for those with IBS is implementing a low FODMAP diet 43 , Similarly, athletes commonly reported using a low FODMAP diet to manage Ex-GIS successfully in the current study.
A low FODMAP diet has also reportedly been used to manage Ex-GIS in other investigations with athletes 25 , However, the time frame for implementation in previous studies, including the current study, has not been investigated.
It has been shown that a short-term h low FODMAP diet before endurance exercise can reduce Ex-GIS and malabsorption 10 , and the impact of this short-term dietary restriction on overall nutritional status is unlikely to be significant.
However, a low FODMAP diet is not designed to be followed long-term due to possible nutritional deficiencies if chronically administered 43 , However, if a low FODMAP diet is necessary over more extended time frames i.
Dairy avoidance around exercise was an exclusive dietary strategy employed by athletes to reduce Ex-GIS, i. In this study, dairy products were avoided before, during, and after exercise. Endurance athletes may have been avoiding dairy products due to the lactose component, which is typically moderated on a low FODMAP diet It is possible that endurance athletes avoid dairy as they have lactose intolerance.
Lactose intolerance is one of the most commonly reported food intolerances, with many symptoms overlapping with IBS, e. In the current study, implementing a lactose-free diet was also reported as a successful dietary strategy to manage Ex-GIS; however, slightly lower implementation rates before, during, and after exercise were reported compared to a dairy-free diet.
Future research could also investigate if athletes have food allergies or intolerances, hence the need for a dairy or lactose-free diet.
Many endurance athletes also reported successfully increasing carbohydrate around endurance exercise to mitigate Ex-GIS development. Increasing carbohydrate intake around exercise is a well-advocated dietary method to facilitate carbohydrate availability, particularly for endurance and ultra-endurance athletes 3 , 29 , However, research has also shown that carbohydrate consumed during exercise facilitates splanchnic region blood flow It is possible this also promotes intestinal epithelial blood flow, reducing epithelium damage.
Indeed, carbohydrate or whey protein hydrolysate WPH ingestion before and during exercise ameliorates intestinal epithelial injury and reduces small intestine permeability 24 , which may also indicate maintenance of splanchnic blood flow.
However, given Ex-GIS severity is greater following WPH ingestion, carbohydrate is likely the preferential macronutrient due to protective mechanisms at the intestinal epithelium, fewer Ex-GIS, and a valuable exogenous fuel source for skeletal muscle A further consideration when increasing carbohydrate intakes is that athletes may inadvertently increase FODMAP loads due to many carbohydrate foods being associated with a higher FODMAP content, e.
Therefore, understanding which foods are rich in carbohydrates but lower in FODMAP content is a dietary strategy some athletes who experience Ex-GIS may have to learn how to apply.
It is acknowledged that some athletes used a combination of dietary and non-dietary strategies to manage Ex-GIS and a smaller proportion used non-dietary strategies exclusively.
Relaxation and psychological interventions such as cognitive behavior therapy, hypnotherapy, or psychological therapy are recommended strategies for people with IBS These non-dietary interventions have the potential to reduce Ex-GIS in endurance athletes, but further research is required to confirm efficacy.
Observational studies indicate that there may be a link between anxiety or stress and GIS development in athletes 50 , but the effect on both functional e. Further research using randomized controlled cross-over or parallel trials into the use of non-dietary approaches as a strategy to manage Ex-GIS is required, in addition to applying recommended methodological approaches Given the subtleties of negotiating the most popular dietary strategies for managing Ex-GIS against current sports nutrition guidelines for endurance athletes, it is perhaps not surprising to learn that athletes mainly source support from accredited practicing dietitians.
Accredited practicing dietitians are uniquely skilled in addressing health and performance-related dietary requirements. It is suggested that an accredited practicing dietitian experienced in sports nutrition and the administration of acute dietary strategies for managing Ex-GIS be sought Specific nutritional guidance should be provided to individuals who experience Ex-GIS, especially regarding carbohydrate loading and acute carbohydrate intakes before and during exercise.
During training, dietary strategies should be trialed to establish individual tolerance and self-perceived efficacy before considering implementation during competition, e.
One of the limitations of the current study is that convenience sampling was applied, i. As all researchers were registered dietitians, it is possible that athletes had a better appreciation of the importance of receiving therapeutic dietary advice through a registered health professional.
It is also possible that athletes were from countries with greater access to a registered dietitian, e. non-western countries 47 , which may over-represent accredited practicing dietitians as the most common source of nutrition information for managing Ex-GIS. Endurance athletes try different dietary methods to manage Ex-GIS, e.
All of these dietary strategies tried were rated as successful. This is especially applicable for accredited practicing dietitians whom athletes often seek for nutrition information to manage Ex-GIS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee—University of the Sunshine Coast, Australia ethics approval number: S RS and GS: conceptualization and writing—original draft.
RS, GS, RC, FP, and DL: methodology and writing—review and editing. RS: statistical analysis and project administration. GS: supervision. All authors contributed to the article and approved the submitted version. It is acknowledged that RS has received an Australian Government Research Training Program Scholarship for her research studies.
The support from the University of the Sunshine Coast and the Australian Government is gratefully received. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Deutsche Ultramarathon-Vereinigung. Ultra Marathon Statistics. org accessed April 9, Google Scholar. Costa RJS, Gaskell SK, McCubbin AJ, Snipe RMJ.
Exertional-heat stress-associated gastrointestinal perturbations during olympic sports: management strategies for athletes preparing and competing in the Tokyo olympic games. Temperature Austin. doi: PubMed Abstract CrossRef Full Text Google Scholar. Costa RJS, Hoffman MD, Stellingwerff T.
Considerations for ultra-endurance activities: part 1- nutrition. Res Sports Med. Costa RJS, Knechtle B, Tarnopolsky M, Hoffman MD. Nutrition for ultramarathon running: trail, track, and road. Int J Sport Nutr Exerc Metab. Costa RJS, Snipe RMJ, Kitic CM, Gibson PR.
Systematic review: exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment Pharmacol Ther. Gaskell SK, Rauch C, Costa RJS. Gastrointestinal assessment and management procedures for exercise-associated symptoms.
Aspetar Sp Med J. Costa RJS, Miall A, Khoo A, Rauch C, Snipe R, Camoes-Costa V, et al. Gut-training: the impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance.
Appl Physiol Nutr Metab. Miall A, Khoo A, Rauch C, Snipe RMJ, Camoes-Costa VL, Gibson PR, et al. Two weeks of repetitive gut-challenge reduce exercise-associated gastrointestinal symptoms and malabsorption.
Scand J Med Sci Sports. Costa RJ, Snipe R, Camoes-Costa V, Scheer V, Murray A. The impact of gastrointestinal symptoms and dermatological injuries on nutritional intake and hydration status during ultramarathon events.
Sports Med Open. Gaskell SK, Taylor B, Muir J, Costa RJS. Impact of h high and low fermentable oligo-, di-, monosaccharide, and polyol diets on markers of exercise-induced gastrointestinal syndrome in response to exertional heat stress.
Rauch CE, McCubbin AJ, Gaskell SK, Costa RJS. Feeding tolerance, glucose availability, and whole-body total carbohydrate and fat oxidation in male endurance and ultra-endurance runners in response to prolonged exercise, consuming a habitual mixed macronutrient diet and carbohydrate feeding during exercise.
Front Physiol. Gaskell SK, Rauch CE, Costa RJS. Gastrointestinal assessment and therapeutic intervention for the management of exercise-associated gastrointestinal symptoms: a case series translational and professional practice approach.
Gaskell SK, Snipe RMJ, Costa RJS. Test-retest reliability of a modified visual analog scale assessment tool for determining incidence and severity of gastrointestinal symptoms in response to exercise stress. Stuempfle KJ, Hoffman MD. Gastrointestinal distress is common during a km ultramarathon.
J Sports Sci. Snipe RMJ, Costa RJS. Does biological sex impact intestinal epithelial injury, small intestine permeability, gastrointestinal symptoms and systemic cytokine profile in response to exertional-heat stress?
Costa RJS, Camoes-Costa V, Snipe RMJ, Dixon D, Russo I, Huschtscha Z. Impact of exercise-induced hypohydration on gastrointestinal integrity, function, symptoms, and systemic endotoxin and inflammatory profile.
J Appl Physiol Costa RJ, Gill SK, Hankey J, Wright A, Marczak S. Perturbed energy balance and hydration status in ultra-endurance runners during a 24 h ultra-marathon. Br J Nutr. Bennett CJ, Henry R, Snipe RMJ, Costa RJS. Is the gut microbiota bacterial abundance and composition associated with intestinal epithelial injury, systemic inflammatory profile, and gastrointestinal symptoms in response to exertional-heat stress?
J Sci Med Sport. Snipe RMJ, Khoo A, Kitic CM, Gibson PR, Costa RJS. The impact of mild heat stress during prolonged running on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profiles.
Int J Sports Med. The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Eur J Appl Physiol. Gaskell SK, Rauch CE, Parr A, Costa RJS.
Diurnal versus nocturnal exercise-effect on the gastrointestinal tract. Med Sci Sports Exerc. Hoffman MD, Fogard K.
Journal of encurance International Cauliflower mac and cheese of Sports Nutrition volume 17Article gasrointestinal 32 Cite this article. Metrics details. Endurance runners Cauliflower mac and cheese experience exercise-induced enduance GI symptoms, negatively enduranxe their performance. A questionnaire designed to assess dietary restrictions pre-racing and gastrointestinal symptoms was administered to runners. Rates of food avoidance were elevated in younger and more competitive runners. The prevalence of GI symptoms was higher in younger athletes, especially females, which may explain their propensity to avoid foods. Lower recreational athletes were the least likely to report GI symptoms.This is enduramce third of a series of 3 posts on gastro-intestinal problems. Unfortunately, GI-problems Elegant Managing gastrointestinal distress during endurance events common, especially amongst endurance athletes and gastrointestinwl can easily ruin a race Read here.
The symptoms are diverse and so are the causes Read here. However, there appear eventw be a number of ways to reduce the dishress.
Not all duing these fastrointestinal may work for everyone, but hopefully every sufferer can find ebdurance or Fat oxidation training ways out of this gastrointestina that will work for them.
The endurancr below distrews based durinb limited research, but anecdotally gastrointeatinal guidelines Managjng to be effective:. Gastrountestinal high Mabaging foods in the durkng or Managing gastrointestinal distress during endurance events days before competition.
For the athlete in training, a diet with Managng fiber will help to evdnts Managing gastrointestinal distress during endurance events bowel regular. Fiber before race day is Managing gastrointestinal distress during endurance events.
By definition, fiber is not digestible, so Managing gastrointestinal distress during endurance events fiber that is eaten essentially passes distresss the Caloric intake recommendation tract. Increased bowel durign during exercise are not desirable and will accelerate fluid loss.
It may also result endjrance unnecessary gas production which durkng cause cramping. Especially Caloric expenditure tracker those individuals who are prone to develop GI-symptoms a evvents fiber diet the day before or even a couple of days djring is recommended.
Choose endirance white foods, like regular pasta, white rice, and plain bagels gasttrointestinal of whole grain bread, high fiber cereals, oats and endurancs rice.
Encurance the food labels endurancw fiber content. Most fruits and vegetables are Managing gastrointestinal distress during endurance events endjrance fiber but there are gatsrointestinal few exceptions: zucchini, tomatoes, olives, grapes, and grapefruit Muscle definition exercises have less than durlng gram of fiber bastrointestinal serving.
Avoid aspirin distrsss non-steroidal tastrointestinal drugs NSAIDs such as ibuprofen. Gastroijtestinal aspirin and NSAIDs are commonly have been shown to increase intestinal permeability and may increase the incidence of GI complaints.
The enddurance of NSAIDs in the pre-race period should be discouraged. Avoid Manaaging products that contain Cauliflower mac and cheese as even mild lactose Managlng can cause problems during Easy and effective weight loss. For instance, it is xuring to avoid milk completely or get lactose free milk.
Soy, rice, and almond milks generally don't contain lactose. Avoid high fructose foods in particular drinks that have exclusively fructose. Fructose is not only found in fruit, but also in most processed sweets; candy, cookies etc.
Some fruit juices are almost exclusively fructose. Fructose is absorbed by the intestines more slowly the tolerance of fructose is much less than glucose may lead to cramping, loose stool and diarrhea.
Having said that, I have discussed before that fructose in combination with glucose may not cause problems and may even be better tolerated see the full blog here. Since dehydration can exacerbate GI-symptoms it is important to avoid dehydration.
Start the race well hydrated. Make sure to experiment with your pre-race and race-day nutrition plan many times prior to race day.
This will allow you to figure out what does and does not work for you, and to reduce the chances that GI issues will ruin your race. Training the gut is another practice.
If your gut is adapted to the foods you consume during a race, you are less likely to get stomach problems. If you are avoiding carbohydrate in daily life, your intestines will respond by reducing intestinal transporter numbers so your ability to absorb carbohydrate is reduced.
On race day you may not be able to absorb all of the ingested and this may cause GI-issues. The advice is therefore not to restrict carbohydrate intake and regularly consume carbohydrate during training.
Read more about training your gut here. de Oliveira, E. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med 44 Suppl 1: S This full reference can be downloaded FREE here. Are extreme glycogen loading protocols necessary?
Does collagen strengthen connective tissue in muscle? Is fructose bad for health? The optimal ratio of carbohydrates. Does dehydration reduce performance? Iron infusion or injection for athletes. If you want to find out the best types of protein, optimal amounts, or timing.
Click here. Want to know more about nutrition for running. If you want to know more about supplements, the benefits and the risks. General sports nutrition topics can be found here. top of page. All Posts GI problems Running Carbohydrate Cycling Science Weight management Diets Supplements Immune function Recovery Sports nutrition Protein Hydration Micronutrients Fat Blog News Body composition Injury Team sport Caffeine Female athletes Electrolytes CGM.
Asker Jeukendrup 3 min read. Prevention of gastro-intestinal problems in athletes. Avoid high fiber foods. Avoid aspirin and non-steroidal anti-inflammatory drugs NSAIDs. Avoid milk products.
Avoid fructose-only foods. Avoid dehydration. Practice new nutrition strategies. Train your gut. Recent Posts See All. Post not marked as liked 4. Post not marked as liked 1. Post not marked as liked All Posts posts GI problems 29 29 posts Running 24 24 posts Carbohydrate 64 64 posts Cycling 28 28 posts Science 46 46 posts Weight management 22 22 posts Diets 25 25 posts Supplements 57 57 posts Immune function 21 21 posts Recovery 59 59 posts Sports nutrition 88 88 posts Protein 35 35 posts Hydration 26 26 posts Micronutrients 13 13 posts Fat 18 18 posts Blog posts News 14 14 posts Body composition 13 13 posts Injury 11 11 posts Team sport 12 12 posts Caffeine 11 11 posts Female athletes 4 4 posts Electrolytes 10 10 posts CGM 4 4 posts.
carbohydrate performance absorption recovery hydration GI problems protein glucose stomach problems train your gut adaptation caffeine Fat sleep allergies football marathon soccer supplements training body weight breakfast coffee diabetes electrolytes fat fructose glucose monitoring glycogen hypoglycemia insulin lactose men muscle building Protein protein synthesis science sports nutrition women amino acids amylopectin antioxidants beta alanine Bone bone mineral density brain CGM chewing gum circadian rhythm CNS conference creatine cycling dairy daptation dehydration economy efficiency energy availability fatigue female Fibre fish oil Fish oil fluids galactose gastrointestinal problems genetics genomics glycemic index gut health heat HMB hunger.
Sports nutrition. bottom of page.
: Managing gastrointestinal distress during endurance events| References | The questionnaire included participant information e. van Wijck K, Lenaerts K, van Loon LJC, Peters WHM, Buurman WA, Dejong CHC. In addition, Ex-GIS incidence and severity have been shown to impair exercise performance in controlled laboratory experimental models 7 , 8 , Article CAS PubMed Google Scholar Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. References De Oliveira, E. |
| Runner's Stomach: Causes, Treatment & Prevention | If Manqging love nutrition, gastroihtestinal Cauliflower mac and cheese commit to gastrountestinal revenue stream duribg become a eneurance. Potentially these Pomegranate Research are competing at lower intensities, thus have fewer symptoms, Greek yogurt breakfast GI symptoms are Managing gastrointestinal distress during endurance events to increase with exercise intensity [ 825 ]. Open 2, 1— Rates of food avoidance were elevated in younger and more competitive runners. Keep it at this level for long enough to allow your digestive system to adapt. The athlete also experienced severe belching and dizziness during exercise and severe dizziness and nausea post-exercise. Am J Physiol Gastrointest Liver Physiol. |
| Top 10 tips to avoiding gastrointestinal distress during a triathlon | Fortunately, food intolerances were typically clearly identified. With respect to food categories: fats, oils, spicy foods, and high FODMAP foods should be added to future questionnaires. Additionally, we did not ask participants to indicate the severity of their symptoms or provide a symptom for each food avoidance; thus, we cannot associate a specific food to a specific symptom or comment on the degree of discomfort. The identification of food avoidance trends will direct future clinical trials designed to identify specific foods endurance runners can consume to minimize GI symptoms and optimize performance. The questionnaire is available as a supplemental file see Additional file 1. de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sport Med. Gil SM, Yazaki E, Evans DF. Aetiology of running-related gastrointestinal dysfunction: how far is the finishing line? Sports Med. Article CAS Google Scholar. Stuempfle KJ, Hoffman MD. Gastrointestinal distress is common during a km ultramarathon. J Sports Sci. Waterman JJ, Kapur R. Upper gastrointestinal issues in athletes. Curr Sports Med Rep. Pugh JN, Sparks AS, Doran DA, Fleming SC, Langan-Evans C, Kirk B, et al. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur J Appl Physiol. Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. Wilson PB. Perceived life stress and anxiety correlate with chronic gastrointestinal symptoms in runners. Costa RJS, Snipe RMJ, Kitic CM, Gibson PR. Systematic review: exercise-induced gastrointestinal syndrome—implications for health and intestinal disease. Aliment Pharmacol Ther. Costa RJS, Hoffman MD, Stellingwerff T. Considerations for ultra-endurance activities: part 1- nutrition. Res Sport Med. Karhu E, Forsgård RA, Alanko L, Alfthan H, Pussinen P, Hämäläinen E, et al. Exercise and gastrointestinal symptoms: running-induced changes in intestinal permeability and markers of gastrointestinal function in asymptomatic and symptomatic runners. Eur J Appl Physiol ; dx. Rehrer NJ, Smets A, Reynaert H, Goes E, de Meirleir K. Effect of exercise on portal vein blood flow in man. Med Sci Sports Exerc. Horner KM, Schubert MM, Desbrow B, Byrne NM, King NA. Acute exercise and gastric emptying: a meta-analysis and implications for appetite control. Gibson PR, Kitic CM, Costa RJS, Snipe RMJ, Khoo A. The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Snipe RMJ, Khoo A, Kitic CM, Gibson PR, Costa RJS. The impact of mild heat stress during prolonged running on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profiles. Int J Sports Med. Tiller NB, Roberts JD, Beasley L, Chapman S, Pinto JM, Smith L, et al. International Society of Sports Nutrition position stand: nutritional considerations for single-stage ultra-marathon training and racing. Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet. Burke LM, Jones AM, Jeukendrup AE, Mooses M. Contemporary nutrition strategies to optimize performance in distance runners and race walkers. Int J Sport Nutr Exerc Metab. Lis DM, Stellingwerff T, Shing CM, Ahuja KDK, Fell JW. Exploring the popularily, experiences, and beliefs surrounding gluten-free diets in nonceliac athletes. Gaskell SK, Costa RJS. Applying a low-FODMAP dietary intervention to a female ultraendurance runner with irritable bowel syndrome during a multistage ultramarathon. Lis DM, Ahuja KDK, Stellingwerff T, Kitic CM, Fell J. Case study: utilizing a low FODMAP diet to combat exercise-induced gastrointestinal symptoms. Wiffin M, Smith L, Antonio J, Johnstone J, Beasley L, Roberts J. Effect of a short-term low fermentable oligiosaccharide, disaccharide, monosaccharide and polyol FODMAP diet on exercise-related gastrointestinal symptoms. Miall A, Khoo A, Rauch C, Snipe RMJ, Camões-Costa VL, Gibson PR, et al. Two weeks of repetitive gut-challenge reduce exercise-associated gastrointestinal symptoms and malabsorption. Scand J Med Sci Sport. Lenth R. Java applets for power and sample size [computer software] [Internet]. Parnell JA, Lafave H, Wagner-Jones K, Madden RF, Erdman KA. Development of a questionnaire to assess dietary restrictions runners use to mitigate gastrointestinal symptoms. Strid H, Simréén M, Störsrud S, Stotzer PO, Sadik R. Effect of heavy exercise on gastrointestinal transit in endurance athletes. Scand J Gastroenterol. Glabska D, Jusinska M. Analysis of the choice of food products and the energy value of diets of female middle-and long-distance runners, depending on the self-assessment of their nutritional habits. Rocz Państwowego Zakładu Hig. Google Scholar. Lis D, Ahuja KDK, Stellingwerff T, Kitic CM, Fell J. Food avoidance in athletes: FODMAP foods on the list. Appl Physiol Nutr Metab. Yantcheva B, Golley S, Topping D, Mohr P. Food avoidance in an Australian adult population sample: the case of dairy products. Public Health Nutr. Bartley J, McGlashan SR. Does milk increase mucus production? Med Hypotheses. Carbohydrate and protein intake during exertional heat stress ameliorates intestinal epithelial injury and small intestine permeability. Gentle HL, Love TD, Howe AS, Black KE. A randomised trial of pre-exercise meal composition on performance and muscle damage in well-trained basketball players. Bronkowska M, Kosendiak A, Orzeł D. Assessment of the frequency of intake of selected sources of dietary fibre among persons competing in marathons. Jang LG, Choi G, Kim SW, Kim BY, Lee S, Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study. Rehrer NJ, van Kemenade M, Meester W, Brouns F, Saris W. Gastrointestinal complaints in relation to dietary intake in triathletes. Int J Sport Nutr. van Wijck K, Lenaerts K, Grootjans J, Wijnands KAP, Poeze M, van Loon LJC, et al. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. Müller M, Canfora EE, Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: modulation by dietary fibers. de Oliveira EP, Burini RC. Food-dependent, exercise-induced gastrointestinal distress. Patel RK, Brouner J, Spendiff O. Dark chocolate supplementation reduces the oxygen cost of moderate intensity cycling. Muller-Lissner SA, Kaatz V, Brandt W, Keller J, Layer P. The perceived effect of various foods and beverages on stool consistency. To date, no study has comprehensively assessed individual gastrointestinal responses to exertional or exertional-heat stress, and implemented an individualised action plan in the prevention and management of GIS during exercise, stemming from EIGS. Establishing the causal and exacerbation factor s for a particular GIS during exercise is difficult without an individual tailored exercise gastrointestinal assessment due to the multifaceted and multilayers of EIGS e. Therefore, the aims of this translational research case series were to: 1 Clinically assess athletes presenting severe GIS during exercise using retrospective exploration; 2 provide a prospective gastrointestinal assessment protocol during exercise GastroAxEx , using previously established valid and reliable gastrointestinal assessment measurement tools that were used to inform an individualised therapeutic intervention for EIGS and GIS during exercise; 3 implement individualised therapeutic management interventions; and 4 assess outcomes of therapeutic management plans in training and competition, and adjust accordingly. After ethics approval from Monash University Human Research Ethics Committee , 12 athletes presented to the BASE Facility, Nutrition and Exercise Clinic, at Monash University Melbourne, Australia with GIS during exercise experienced during competition. However, nine recreational to elite level non-heat acclimatised endurance athletes met the inclusion criteria, provided informed consent, and volunteered to participate in the translational research. Each of the athletes were identified as experiencing EIGS and subsequent GIS during exercise Table 1. Table 1. Case series and control participant characteristics and presentation of exercise-associated gastrointestinal symptoms GIS. EIGS and GIS during exercise assessment and management procedures have previously been described in Gaskell et al. Figure 1. Four-phase EIGS and GIS during exercise assessment and management procedures. Ax, assessment; GastroAxEx, gastrointestinal assessment during exercise; GIS, gastrointestinal symptoms; HR, heart rate; RPE, rating of perceived exertion; TCR, thermal comfort rating; T re , rectal temperature. Intervention based on original research providing evidence of positive outcomes, and avoiding those showing evidence of neutral to negative outcomes, on markers of EIGS e. Adapted from Gaskell et al. Phase 1: Clinical assessment of the athlete including specific details about their GIS during exercise i. Suspected EIGS was identified if athletes met the following criteria: 1 rapid onset of nausea, urge to regurgitate, regurgitation at later stage of competition e. Phase 2: A laboratory-controlled simulated gastrointestinal assessment during exercise GastroAxEx , tailored to the individual, was designed and conducted Table 2. Based on previously published research, the magnitude of exertional stress applied was in accordance with what typically provokes exercise-associated gastrointestinal disturbance of relative performance and clinical significance Costa et al. Based on individual clinical assessment from Phase 1 appropriate gastrointestinal and physiological assessment markers were established and determined. Table 2. Individualised laboratory-controlled simulated gastrointestinal assessment during exercise GastroAxEx in endurance athletes experiencing EIGS with severe GIS and endurance athletes not experiencing EIGS and presenting minimal GIS controls. Intervention may have included the following: h dietary control [i. Phase 4: Monitoring and readjustment of intervention, which included checking the athlete was healthy leading into intervention, followed the dietary intervention, menstrual status where applicable i. Figure 1 part 2 and Table 2 depicts the laboratory controlled GastroAxEx protocol developed for each individual athlete based on their clinical presentation of EIGS and GIS during exercise. A running protocol on a motorised treadmill Forma Run ; Technogym, Seattle, WA, United States was undertaken by seven athletes i. All exercise protocols were at least 2 h duration with the longest 3 h 40 min. Six athletes undertook exercise in the heat ambient temperature 30—35°C and three in thermoneutral conditions ambient temperature 23°C , as indicated by Phase 1. Athletes were asked to replicate their typical event preparation i. Athletes reported to the laboratory 1 h before exercise commencement, after consuming their typical pre-event meal or snack 2 h beforehand. A dietary log 1—3 days determined their nutritional intake, as per previously established dietary intake assessment and analysis procedures Costa et al. Participants were asked to void before nude body mass measurement, provide a breath sample into a mL breath collection bag Wagner Analysen Technick, Bremen, Germany , and complete a mVAS GIS assessment tool Gaskell et al. Blood samples were then collected where indicated by venipuncture from an antecubital vein into lithium heparin 6 mL, 1. Rectal temperature T re was monitored during exercise with participants inserting a thermocouple 12—15 cm beyond the external anal sphincter Alpha Technics Precision Temperature Thermometer, Oceanside, CA, United States. Athletes then completed their individual tailored GastroAxEx Table 2. Participants were provided with and instructed to consume their typical during competition food and fluid intake i. During exercise food and fluid intake was recorded in real-time. Water was available ad libitum throughout exercise. To determine OCTT, participants were provided with a solution containing 20 g of lactulose Actilax, alphapharm, Qld, Australia , in the final 30 min of exercise. Breath samples were then collected immediately post-exercise and every 15 min during the recovery period, ranging from a 2 to 3 h timeline. The time interval between ingestion of lactulose and rise in breath hydrogen H 2 10 ppm, with two consecutive readings above baseline BL was used as a measure of OCTT Bate et al. T re , heart rate HR , rating of perceived exertion RPE , and thermal comfort rating TCR were measured every 30 min during exercise. Body mass, GIS, and feeding tolerance were measured every 30 min during exercise. Breath-by-breath indirect calorimetry Vmax Encore Metabolic Cart, CaseFusion-BD, Franklin Lakes, NJ, United States was used to measure V̇ O 2 , V̇ CO 2 , and RER for 5 min continuously every 20 min during exercise. Total non-protein carbohydrate and fat oxidation was determined from the equations of Péronnet and Massicotte :. Immediately after exercise, a blood sample was collected where indicated. Nude body mass and GIS were recorded immediately post-exercise. Participants remained seated during the recovery period and consumed water ad libitum. GIS were recorded every 30 min during the 2—3 h post-exercise period. Participants were provided with a standard meal 2 h post-exercise in accordance with ethical procedures, as previously described Russo et al. Total body water, including extracellular water, was determined through an 8-point multifrequency bioelectrical impedance analyser mBCA , Seca, Ecomed, Hamburg, Germany before exercise and during the recovery period, where indicated. Breath samples 20 ml were analysed in duplicate CV: 3. Whole blood haemoglobin was determined by a HemoCue system Hb; HemoCue, Ängelholm, Sweden , and haematocrit was determined by the capillary method with a microhematocrit reader ThermoFisher Scientific , both from heparin whole blood samples. Haemoglobin and haematocrit values were used to estimate changes in plasma volume P V relative to baseline and used to correct plasma variables. Blood glucose concentration was measured pre, every 30 min during and post-exercise Accu-Chek Proforma ; Roche Diagnostics, Indianapolis, Ind. The remaining heparin and K 3 EDTA whole blood samples were centrifuged at 4, rpm for 10 min within 15 min of sample collection. Plasma was aliquoted into 1. Plasma concentration of cortisol DKO, IBL International, Kiel, Germany , intestinal fatty acid binding protein I-FABP HK, Hycult Biotech, Uden, Netherlands , soluble CD14 sCD14 HK, Hycult Biotech, Uden, Netherlands , LBP HK, Hycult Biotech, Uden, Netherlands and claudin-3 SEFHu, Cloud-Clone Corp. Plasma concentrations of IL-1β, TNF-α, IL-6, IL-8, IL, and IL-1ra were determined by multiplex ELISA HCYTOMAGK, EMD Millipore, Darmstadt, Germany. From a translational professional practice perspective, data are presented as full values for individual responses for all measured primary and secondary variables. In cases where primary or secondary variables were not collected, the n has been reported accordingly e. Table 2 depicts the physiological and thermoregulatory responses, hydration, blood glucose, and total carbohydrate and fat oxidation rates in response to the GastroAxEx. Table 3 depicts the gastrointestinal integrity and function markers, systemic endotoxin and inflammatory cytokine profiles, GIS during exercise, and feeding tolerance responses to the GastroAxEx. No substantial change was observed pre- to post-exercise in other markers of gastrointestinal integrity, including plasma claudin, sCD14, LBP, and inflammatory cytokine IL-1β, TNF-α, IL-6, IL-8, IL, and IL-1ra concentrations. Only one of the control athletes had their OCTT tested and this was classified as a normal response 60 min. Both of the control cases experienced minimal GIS during and in the post-exercise period. However, tolerance to food i. An interest to drink i. Table 3. Leukocyte trafficking A , stress hormone response, gastrointestinal integrity and functional responses, systemic endotoxin B , inflammatory cytokine profiles C , gastrointestinal symptoms GIS D and feeding tolerance E in response to the gastrointestinal assessment during exercise GastroAxEx in endurance athletes experiencing EIGS with severe GIS and endurance athletes not experiencing EIGS and presenting minimal GIS controls. Figure 2. Qualitative outcomes are depicted in Table 4. Based on GastroAxEx outcomes and in accordance with previous prevention and management strategies Costa et al. Table 4. Exercise induced gastrointestinal syndrome EIGS and exercise-associated gastrointestinal symptoms prevention and management therapeutic intervention and progress outcomes. It should be noted, however, that two of these athletes have only been able to implement the intervention in training due to either injury or not entering competition at the time of completion of this case series. The current translational research case series aimed to: 1 Clinically assess endurance athletes presenting severe GIS during exercise using retrospective exploration; 2 provide a GastroAxEx using previously established valid and reliable gastrointestinal assessment measurement tools which were used to inform an individualised therapeutic intervention for EIGS and associated GIS; 3 implement individualised therapeutic management interventions; and 4 assess outcomes of therapeutic management plans in training and competition, and adjust accordingly. With the exception of two athletes showing intestinal epithelial injury, no substantial disturbance was observed in the circulatory-gastrointestinal pathway of EIGS in the majority of the case series athletes, including gastrointestinal integrity and systemic endotoxin and immune markers. However, there was disturbance to the neuroendocrine-gastrointestinal pathway of EIGS, indicative of reduced OCTT in all the case athletes. These observations suggest functional issues instigated by EIGS are likely culprits of GIS during exercise in the current cohort, and that targetting interventions to improve these debilitating gastrointestinal functional issues are likely to reduce the incidence and severity of GIS during exercise. Conversely, targetting the circulatory-gastrointestinal pathway specifically with interventions that focus on maintaining the integrity of the intestinal epithelial are unlikely to rectify the GIS during exercise. Each of the four-phases play a significant role in the prevention and management of EIGS and associated GIS during exercise for the athlete. Although the most common reported GIS during competition in the clinical assessment Phase 1 was nausea and vomiting, negatively influencing feeding tolerance during exercise, two athletes experienced feeding intolerance due to general exercise fatigue accumulation as competition progressed. Some athletes were prone to experience GIS during exercise in the heat, whereas others experienced GIS during exercise in temperate ambient conditions. It is therefore imperative that the sports dietetic or medical practitioner collect comprehensive clinical information of the athlete presenting with EIGS and associated GIS in order to inform the GastroAxEx to best match the scenario resulting in GIS during exercise. A substantial amount of both laboratory and field, exploratory or intervention, exercise gastroenterology research has shown large individual variation in EIGS perturbations such as gastrointestinal integrity, function, and systemic markers that can influence GIS during exercise outcomes such as incidence, type, and severity Gill et al. Therefore, it is important that in phase 2 the laboratory controlled GastroAxEx is individualised, as this will subsequently have flow on effects to Phase 3 informing prevention and management strategies of EIGS and associated GIS during exercise. For example, there is individual variability in gastrointestinal function and GIS during diurnal and nocturnal exercise Gaskell et al. Other athletes may have varying feeding tolerance levels that require an individualised gut challenge protocol. In the current athlete case series the following outcomes were observed: 1 The athlete that presented the greatest intestinal epithelial injury i. Proposed Δ pre- to post-exercise plasma I-FABP concentration reflecting magnitude of clinical relevance i. The athlete also experienced severe belching and dizziness during exercise and severe dizziness and nausea post-exercise. unit attributed to a pronounced anti-inflammatory response i. After exercise this athlete experienced projectile vomiting, nausea, belching and urge to regurgitate. unit Snipe et al. The two control cases that presented minimal GIS during and in the post-exercise period also presented with minimal disturbance to gastrointestinal integrity, function where measured , systemic endotoxaemia and inflammatory profile response; but magnitude of responses were of no clinical consequence and not sufficient to impede exercise workload and warrant exercise cessation or withdrawal. In comparison, previous laboratory exertional stress models, all study participants have completed the exercise protocol Costa et al. All participants in this case series experienced a delayed OCTT response, this is in contrast to Gaskell et al. In contrast, the exercise stress model in Gaskell et al. Due to large individual variation in factors exacerbating GIS during exercise in athletes there is no one standard approach and therapeutic intervention. Other examples of nutrition supplementation lacking evidence in this area are amino acids e. When designing the GastroAxEx, it may not always be possible to identically replicate the exercise i. For example, one of the case series athletes experienced EIGS during enduro-motorcycling events, suggested to be due to dehydration and heat stress. The athlete also participated in marathon running events. Therefore, a running exertional model was used to induce similar dehydration and thermoregulatory strain due to the logistics of simulating an enduro-motorcycling bout in laboratory-controlled conditions. When conducting the GastroAxEx, it is important to mimic the real-life scenario as close as possible leading up to EIGS and GIS during exercise i. This will then help identify the main causal and exacerbation factors of EIGS, and subsequent GIS during exercise, which will inform the individualised therapeutic intervention for the prevention and management of EIGS and GIS during exercise. Phase 3 involves an individualised therapeutic intervention targetted at EIGS and GIS during exercise, that informed by the GastroAxEx. Once the causal and exacerbator factors are identified the intervention can be determined. Without an understanding of the causal pathway the prevention and management strategies will be non-specific and experimental. For example, the professional triathlete trialled a number of different strategies in order to manage his EIGS and associated GIS during exercise, such as a low FODMAP diet, low carbohydrate and high fat diet and changing the type of race nutrition in training and competition without success. Furthermore, the intake of a glucose-fructose combined solution increases GE and fluid delivery when compared with a glucose-only solution. Additionally, the combined sugar attenuates heart-rate increase and results in lower rates of perceived exertion and lower loss of body weight than glucose alone or water [ 43 ]. Moreover, a solution intake with 1. Hypertonic solutions tend to delay water absorption in the intestine as water instead flows into the intestine to dilute the solution before water is absorbed [ 8 ]. Additionally, there is contention as to whether hypertonic solutions reduce the GE rate [ 46 ]. However, energy density is considered to be more important in determining GE when solutions with an osmolality close to those normally found in sports drinks are used [ 8 ]. The rate of fluid absorptions is closely related to the CHO content of drinks with high CHO concentrations, thus compromising fluid delivery. Hence, a balance must be met between the goal of maintaining hydration status and providing CHO to the working muscle [ 8 ]. Slowed gastric emptying associated with high-intensity exercise is further slowed by the consumption of hypertonic carbohydrate beverages, usually given after running [ 38 ]. Gastric emptying is proportionally slowed as the concentration of carbohydrates increases in replacement fluid because of hyperosmolar effects [ 2 ]. Current nutritional recommendations to endurance athletes are generally based on advice to: 1 drink during exercise to prevent excessive dehydration and excessive changes in electrolyte balance and; 2 maintain carbohydrate oxidation rates and plasma glucose concentrations. However, these two aims fluid delivery and carbohydrate delivery can be difficult to reconcile as increasing the CHO content of a beverage to high levels increases the CHO delivery rate, but decreases fluid delivery. Electrolyte imbalance which is commonly referred to as "water intoxication" and results from hyponatremia low plasma sodium due to excessive water intake has occasionally been reported in long-distance triathletes [ 47 ]. The symptoms of hyponatremia are similar to those associated with dehydration and include mental confusion, weakness and fainting. Because the symptoms of hyponatremia are so similar to those of dehydration, that condition may be dangerously misdiagnosed in endurance races athletes. The usual treatment for dehydration is oral and intravenous administration of fluids. If such treatment were to be given to a hyponatremic individual, the consequences could be fatal [ 8 ]. Hyponatremia may occur in a state of euhydration or even dehydration, but it is generally associated with fluid overload [ 47 ] and the cause is the fluid intake higher than sweat rate, that causes dilutional hyponatraemia [ 48 ]. Triathletes may often develop hyponatremia without displaying symptoms [ 8 ]. In order to prevent hyponatremia, avoiding overhydration and informing athletes about the potential dangers of drinking too much water are recommended. When compared with water, a sodium-containing drink attenuated the drop in plasma sodium [ 49 ]. Physiological adaptations to physical exercises lead to blood volume redistribution favoring the working muscle supply with oxygen and energy-yielding substrate as well as the skin for heating dissipation as sweat. Rapid fluid delivery from fluids intake is the goal of oral rehydration solutions and sports drinks, that provide the addition of sodium and carbohydrates to assist the intestinal absorption of water and muscle-glycogen replenishment, respectively. However, sometimes, fluid delivery and carbohydrate delivery are difficult to reconcile as carbohydrate-rich beverages decrease fluid delivery to the gut, thus delaying water absorption and accentuating gut underperfusion. It is necessary to inform athletes about potential dangers of drinking too much water, advise them to refrain from using hypertonic fluid replacements. Burini FHP, de Oliveira EP, Burini RC: Metabolic Mal Adaptations to Training Continuum-Misconceptions of Terminology and Diagnosis. Rev Bras Med Esporte. Article Google Scholar. Wittbrodt ET: Maintaining fluid and electrolyte balance during exercise. Journal of Pharmacy Practice. de Oliveira EP, Burini RC: The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. Article PubMed Google Scholar. Choi JH, Lee HB, Ahn IS, Park CW, Lee CH: Wheat-dependent, Exercise-induced Anaphylaxis: A Successful Case of Prevention with Ketotifen. Ann Dermatol. Article PubMed Central PubMed Google Scholar. Fujii H, Kambe N, Fujisawa A, Kohno K, Morita E, Miyachi Y: Food-dependent exercise-induced anaphylaxis induced by low dose aspirin therapy. Allergol Int. Article CAS PubMed Google Scholar. Rehrer NJ, Brouns F, Beckers EJ, Frey WO, Villiger B, Riddoch CJ, Menheere PP, Saris WH: Physiological changes and gastro-intestinal symptoms as a result of ultra-endurance running. Eur J Appl Physiol Occup Physiol. Qamar MI, Read AE: Effects of exercise on mesenteric blood flow in man. Article PubMed Central CAS PubMed Google Scholar. Jeukendrup AE, Jentjens RL, Moseley L: Nutritional considerations in triathlon. Sports Med. Jeukendrup AE, Vet-Joop K, Sturk A, Stegen JH, Senden J, Saris WH, Wagenmakers AJ: Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci Lond. Article CAS Google Scholar. Rehrer NJ, van Kemenade M, Meester W, Brouns F, Saris WH: Gastrointestinal complaints in relation to dietary intake in triathletes. Int J Sport Nutr. CAS PubMed Google Scholar. Oktedalen O, Lunde OC, Opstad PK, Aabakken L, Kvernebo K: Changes in the gastrointestinal mucosa after long-distance running. Scand J Gastroenterol. Shadick NA, Liang MH, Partridge AJ, Bingham C, Wright E, Fossel AH, Sheffer AL: The natural history of exercise-induced anaphylaxis: survey results from a year follow-up study. J Allergy Clin Immunol. Castells MC, Horan RF, Sheffer AL: Exercise-induced Anaphylaxis. Curr Allergy Asthma Rep. Loibl M, Schwarz S, Ring J, Halle M, Brockow K: Definition of an exercise intensity threshold in a challenge test to diagnose food-dependent exercise-induced anaphylaxis. Orhan F, Karakas T: Food-dependent exercise-induced anaphylaxis to lentil and anaphylaxis to chickpea in a year-old boy. J Investig Allergol Clin Immunol. Morita E, Matsuo H, Chinuki Y, Takahashi H, Dahlstrom J, Tanaka A: Food-dependent exercise-induced anaphylaxis -importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Bito T, Kanda E, Tanaka M, Fukunaga A, Horikawa T, Nishigori C: Cows milk-dependent exercise-induced anaphylaxis under the condition of a premenstrual or ovulatory phase following skin sensitization. Barg W, Wolanczyk-Medrala A, Obojski A, Wytrychowski K, Panaszek B, Medrala W: Food-dependent exercise-induced anaphylaxis: possible impact of increased basophil histamine releasability in hyperosmolar conditions. Castells MC, Horan RF, Sheffer AL: Exercise-induced anaphylaxis EIA. Clin Rev Allergy Immunol. Kato Y, Nagai A, Saito M, Ito T, Koga M, Tsuboi R: Food-dependent exercise-induced anaphylaxis with a high level of plasma noradrenaline. J Dermatol. Porcel S, Sanchez AB, Rodriguez E, Fletes C, Alvarado M, Jimenez S, Hernandez J: Food-dependent exercise-induced anaphylaxis to pistachio. Galbo H: The hormonal response to exercise. Proc Nutr Soc. Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness. Maresh CM, Gabaree-Boulant CL, Armstrong LE, Judelson DA, Hoffman JR, Castellani JW, Kenefick RW, Bergeron MF, Casa DJ: Effect of hydration status on thirst, drinking, and related hormonal responses during low-intensity exercise in the heat. J Appl Physiol. Coris EE, Ramirez AM, Van Durme DJ: Heat illness in athletes: the dangerous combination of heat, humidity and exercise. Evans GH, Shirreffs SM, Maughan RJ: Postexercise rehydration in man: the effects of carbohydrate content and osmolality of drinks ingested ad libitum. Appl Physiol Nutr Metab. Casa DJ, Armstrong LE, Hillman SK, Montain SJ, Reiff RV, Rich BS, Roberts WO, Stone JA: National Athletic Trainers' Association Position Statement: Fluid Replacement for Athletes. J Athl Train. PubMed Central CAS PubMed Google Scholar. Convertino VA, Armstrong LE, Coyle EF, Mack GW, Sawka MN, Senay LC, Sherman WM: American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. Bouchama A, Knochel JP: Heat stroke. N Engl J Med. van Nieuwenhoven MA, Vriens BE, Brummer RJ, Brouns F: Effect of dehydration on gastrointestinal function at rest and during exercise in humans. Eur J Appl Physiol. Do KD, Bellabarba C, Bhananker SM: Exertional rhabdomyolysis in a bodybuilder following overexertion: a possible link to creatine overconsumption. Clin J Sport Med. Groeneveld GJ, Beijer C, Veldink JH, Kalmijn S, Wokke JH, van den Berg LH: Few adverse effects of long-term creatine supplementation in a placebo-controlled trial. Int J Sports Med. Gualano B, Ugrinowitsch C, Novaes RB, Artioli GG, Shimizu MH, Seguro AC, Harris RC, Lancha AH: Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Leiper JB, Broad NP, Maughan RJ: Effect of intermittent high-intensity exercise on gastric emptying in man. Rehrer NJ, Beckers EJ, Brouns F, ten Hoor F, Saris WH: Effects of dehydration on gastric emptying and gastrointestinal distress while running. van Deventer S, Gouma D: Bacterial translocation and endotoxin transmigration in intestinal ischaemia and reperfusion. Curr Opinion Aneasth. Brock-Utne JG, Gaffin SL, Wells MT, Gathiram P, Sohar E, James MF, Morrell DF, Norman RJ: Endotoxaemia in exhausted runners after a long-distance race. S Afr Med J. Casey E, Mistry DJ, MacKnight JM: Training room management of medical conditions: sports gastroenterology. Clin Sports Med. Wright H, Collins M, Schwellnus MP: Gastrointestinal GIT symptoms in athletes: A review of risk factors associated with the development of GIT symptoms during exercise. International SportMed Journal. Google Scholar. Jeukendrup AE, Currell K, Clarke J, Cole J, Blannin AK: Effect of beverage glucose and sodium content on fluid delivery. |
| Background | Your gut will respond to how you fuel during exercise, and you can train your gut much like any other muscle in the body. Gradually increase your carbohydrate intake and monitor how your body responds so you know what to expect on race day. Anxiety is known to trigger GI distress. Have a strategy to hand that you know can help you manage your pre-race nerves and anxiety. Breathing exercises are a popular strategy on the start line! Consider front loading your nutrition on the bike, there are more opportunities to carry nutrition on the bike and for most, it is easier to consume nutrition on the bike than when running. Try probiotic supplements. Taking probiotic supplements for 12 weeks before a long-distance triathlon has been shown to reduce GI symptoms, or even after 28 days before a marathon Pugh et al, Familiarise yourself with the route, so you know where the aid stations are and toilets if you need them! As well as avoiding high-fibre foods in the days leading up to your race, you could also try low FODMAP before competition. FODMAPs are short-chain carbohydrates found in a wide range of foods that can be poorly absorbed and increase the osmotic load on your small intestine. There is some evidence to support following a low FODMAP diet before a competition can minimise exercise-associated GI symptoms. Last but not least, listen to your body! Pay attention to early signs of GI distress like bloating, cramping, or nausea. If you feel discomfort, slow down, take a walk break, slowing down or taking a break is better than a DNF! Additionally, there is contention as to whether hypertonic solutions reduce the GE rate [ 46 ]. However, energy density is considered to be more important in determining GE when solutions with an osmolality close to those normally found in sports drinks are used [ 8 ]. The rate of fluid absorptions is closely related to the CHO content of drinks with high CHO concentrations, thus compromising fluid delivery. Hence, a balance must be met between the goal of maintaining hydration status and providing CHO to the working muscle [ 8 ]. Slowed gastric emptying associated with high-intensity exercise is further slowed by the consumption of hypertonic carbohydrate beverages, usually given after running [ 38 ]. Gastric emptying is proportionally slowed as the concentration of carbohydrates increases in replacement fluid because of hyperosmolar effects [ 2 ]. Current nutritional recommendations to endurance athletes are generally based on advice to: 1 drink during exercise to prevent excessive dehydration and excessive changes in electrolyte balance and; 2 maintain carbohydrate oxidation rates and plasma glucose concentrations. However, these two aims fluid delivery and carbohydrate delivery can be difficult to reconcile as increasing the CHO content of a beverage to high levels increases the CHO delivery rate, but decreases fluid delivery. Electrolyte imbalance which is commonly referred to as "water intoxication" and results from hyponatremia low plasma sodium due to excessive water intake has occasionally been reported in long-distance triathletes [ 47 ]. The symptoms of hyponatremia are similar to those associated with dehydration and include mental confusion, weakness and fainting. Because the symptoms of hyponatremia are so similar to those of dehydration, that condition may be dangerously misdiagnosed in endurance races athletes. The usual treatment for dehydration is oral and intravenous administration of fluids. If such treatment were to be given to a hyponatremic individual, the consequences could be fatal [ 8 ]. Hyponatremia may occur in a state of euhydration or even dehydration, but it is generally associated with fluid overload [ 47 ] and the cause is the fluid intake higher than sweat rate, that causes dilutional hyponatraemia [ 48 ]. Triathletes may often develop hyponatremia without displaying symptoms [ 8 ]. In order to prevent hyponatremia, avoiding overhydration and informing athletes about the potential dangers of drinking too much water are recommended. When compared with water, a sodium-containing drink attenuated the drop in plasma sodium [ 49 ]. Physiological adaptations to physical exercises lead to blood volume redistribution favoring the working muscle supply with oxygen and energy-yielding substrate as well as the skin for heating dissipation as sweat. Rapid fluid delivery from fluids intake is the goal of oral rehydration solutions and sports drinks, that provide the addition of sodium and carbohydrates to assist the intestinal absorption of water and muscle-glycogen replenishment, respectively. However, sometimes, fluid delivery and carbohydrate delivery are difficult to reconcile as carbohydrate-rich beverages decrease fluid delivery to the gut, thus delaying water absorption and accentuating gut underperfusion. It is necessary to inform athletes about potential dangers of drinking too much water, advise them to refrain from using hypertonic fluid replacements. Burini FHP, de Oliveira EP, Burini RC: Metabolic Mal Adaptations to Training Continuum-Misconceptions of Terminology and Diagnosis. Rev Bras Med Esporte. Article Google Scholar. Wittbrodt ET: Maintaining fluid and electrolyte balance during exercise. Journal of Pharmacy Practice. de Oliveira EP, Burini RC: The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. Article PubMed Google Scholar. Choi JH, Lee HB, Ahn IS, Park CW, Lee CH: Wheat-dependent, Exercise-induced Anaphylaxis: A Successful Case of Prevention with Ketotifen. Ann Dermatol. Article PubMed Central PubMed Google Scholar. Fujii H, Kambe N, Fujisawa A, Kohno K, Morita E, Miyachi Y: Food-dependent exercise-induced anaphylaxis induced by low dose aspirin therapy. Allergol Int. Article CAS PubMed Google Scholar. Rehrer NJ, Brouns F, Beckers EJ, Frey WO, Villiger B, Riddoch CJ, Menheere PP, Saris WH: Physiological changes and gastro-intestinal symptoms as a result of ultra-endurance running. Eur J Appl Physiol Occup Physiol. Qamar MI, Read AE: Effects of exercise on mesenteric blood flow in man. Article PubMed Central CAS PubMed Google Scholar. Jeukendrup AE, Jentjens RL, Moseley L: Nutritional considerations in triathlon. Sports Med. Jeukendrup AE, Vet-Joop K, Sturk A, Stegen JH, Senden J, Saris WH, Wagenmakers AJ: Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci Lond. Article CAS Google Scholar. Rehrer NJ, van Kemenade M, Meester W, Brouns F, Saris WH: Gastrointestinal complaints in relation to dietary intake in triathletes. Int J Sport Nutr. CAS PubMed Google Scholar. Oktedalen O, Lunde OC, Opstad PK, Aabakken L, Kvernebo K: Changes in the gastrointestinal mucosa after long-distance running. Scand J Gastroenterol. Shadick NA, Liang MH, Partridge AJ, Bingham C, Wright E, Fossel AH, Sheffer AL: The natural history of exercise-induced anaphylaxis: survey results from a year follow-up study. J Allergy Clin Immunol. Castells MC, Horan RF, Sheffer AL: Exercise-induced Anaphylaxis. Curr Allergy Asthma Rep. Loibl M, Schwarz S, Ring J, Halle M, Brockow K: Definition of an exercise intensity threshold in a challenge test to diagnose food-dependent exercise-induced anaphylaxis. Orhan F, Karakas T: Food-dependent exercise-induced anaphylaxis to lentil and anaphylaxis to chickpea in a year-old boy. J Investig Allergol Clin Immunol. Morita E, Matsuo H, Chinuki Y, Takahashi H, Dahlstrom J, Tanaka A: Food-dependent exercise-induced anaphylaxis -importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Bito T, Kanda E, Tanaka M, Fukunaga A, Horikawa T, Nishigori C: Cows milk-dependent exercise-induced anaphylaxis under the condition of a premenstrual or ovulatory phase following skin sensitization. Barg W, Wolanczyk-Medrala A, Obojski A, Wytrychowski K, Panaszek B, Medrala W: Food-dependent exercise-induced anaphylaxis: possible impact of increased basophil histamine releasability in hyperosmolar conditions. Castells MC, Horan RF, Sheffer AL: Exercise-induced anaphylaxis EIA. Clin Rev Allergy Immunol. Kato Y, Nagai A, Saito M, Ito T, Koga M, Tsuboi R: Food-dependent exercise-induced anaphylaxis with a high level of plasma noradrenaline. J Dermatol. Porcel S, Sanchez AB, Rodriguez E, Fletes C, Alvarado M, Jimenez S, Hernandez J: Food-dependent exercise-induced anaphylaxis to pistachio. Galbo H: The hormonal response to exercise. Proc Nutr Soc. Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness. Maresh CM, Gabaree-Boulant CL, Armstrong LE, Judelson DA, Hoffman JR, Castellani JW, Kenefick RW, Bergeron MF, Casa DJ: Effect of hydration status on thirst, drinking, and related hormonal responses during low-intensity exercise in the heat. J Appl Physiol. Coris EE, Ramirez AM, Van Durme DJ: Heat illness in athletes: the dangerous combination of heat, humidity and exercise. Evans GH, Shirreffs SM, Maughan RJ: Postexercise rehydration in man: the effects of carbohydrate content and osmolality of drinks ingested ad libitum. Appl Physiol Nutr Metab. Casa DJ, Armstrong LE, Hillman SK, Montain SJ, Reiff RV, Rich BS, Roberts WO, Stone JA: National Athletic Trainers' Association Position Statement: Fluid Replacement for Athletes. J Athl Train. PubMed Central CAS PubMed Google Scholar. Convertino VA, Armstrong LE, Coyle EF, Mack GW, Sawka MN, Senay LC, Sherman WM: American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. Bouchama A, Knochel JP: Heat stroke. N Engl J Med. van Nieuwenhoven MA, Vriens BE, Brummer RJ, Brouns F: Effect of dehydration on gastrointestinal function at rest and during exercise in humans. Eur J Appl Physiol. Do KD, Bellabarba C, Bhananker SM: Exertional rhabdomyolysis in a bodybuilder following overexertion: a possible link to creatine overconsumption. Clin J Sport Med. Groeneveld GJ, Beijer C, Veldink JH, Kalmijn S, Wokke JH, van den Berg LH: Few adverse effects of long-term creatine supplementation in a placebo-controlled trial. Int J Sports Med. Gualano B, Ugrinowitsch C, Novaes RB, Artioli GG, Shimizu MH, Seguro AC, Harris RC, Lancha AH: Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Leiper JB, Broad NP, Maughan RJ: Effect of intermittent high-intensity exercise on gastric emptying in man. Rehrer NJ, Beckers EJ, Brouns F, ten Hoor F, Saris WH: Effects of dehydration on gastric emptying and gastrointestinal distress while running. van Deventer S, Gouma D: Bacterial translocation and endotoxin transmigration in intestinal ischaemia and reperfusion. Curr Opinion Aneasth. Brock-Utne JG, Gaffin SL, Wells MT, Gathiram P, Sohar E, James MF, Morrell DF, Norman RJ: Endotoxaemia in exhausted runners after a long-distance race. S Afr Med J. Casey E, Mistry DJ, MacKnight JM: Training room management of medical conditions: sports gastroenterology. Clin Sports Med. Wright H, Collins M, Schwellnus MP: Gastrointestinal GIT symptoms in athletes: A review of risk factors associated with the development of GIT symptoms during exercise. International SportMed Journal. Google Scholar. Jeukendrup AE, Currell K, Clarke J, Cole J, Blannin AK: Effect of beverage glucose and sodium content on fluid delivery. Nutr Metab Lond. Backx K, van Someren KA, Palmer GS: One hour cycling performance is not affected by ingested fluid volume. Int J Sport Nutr Exerc Metab. PubMed Google Scholar. Bosenberg, A. Strenuous exercise causes systemic endotoxemia. Brock-Utne, J. Endotoxemia in exhausted runners after a long-distance race. Costa, R. Impact of exercise-induced hypohydration on gastrointestinal integrity, function, symptoms, and systemic endotoxin and inflammatory profile. The impact of a dairy milk recovery beverage on bacterially stimulated neutrophil function and gastrointestinal tolerance in response to hypohydration inducing exercise stress. Sport Nutr. Exertional-heat stress-associated gastrointestinal perturbations during Olympic sports: Management strategies for athletes preparing and competing in the Tokyo Olympic Games. Temperature 7, 58— Heat acclimation responses of an ultra-endurance running group preparing for hot desert based competition. Sport Sci. Perturbed energy balance and hydration status in ultra-endurance runners during a 24 h ultra-marathon. Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Systematic review: exercise-induced gastrointestinal syndrome—implications for health and intestinal disease. Aliment Pharmacol. The impact of gastrointestinal symptoms and dermatological injuries on nutritional intake and hydration status during ultramarathon events. Open 2, 1— Compromised energy and nutritional intake of ultra-endurance runners during a multi-stage ultra-marathon conducted in a hot ambient environment. Sports Sci. Water and sodium intake habits and status of ultra-endurance runners during a multi-stage ultra-marathon conducted in a hot ambient environment: An observational study. Gaskell, S. Gastrointestinal assessment and management procedures for exercise-associated gastrointestinal symptoms. Aspetar Sports Med. Diurnal versus Nocturnal Exercise-Impact on the Gastrointestinal Tract. Sports Exerc. Test—retest reliability of a modified visual analog scale assessment tool for determining incidence and severity of gastrointestinal symptoms in response to exercise stress. Impact of h high and low fermentable oligo-, di-, monosaccharide, and polyol diets on markers of exercise-induced gastrointestinal syndrome in response to exertional heat stress. Gill, S. The impact of a hour ultra-marathon on circulatory endotoxin and cytokine profile. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Goulet, E. Effect of exercise-induced dehydration on time-trial exercise performance: a meta-analysis. Grames, C. Ischemic colitis in an endurance runner. Case Rep. Guillochon, M. Solid, gel, and liquid carbohydrate format effects on gut comfortand performance. Haakonssen, E. Dairy-based preexercise meal does not effect gut comfort or time-trial performance in female cyclists. Hoffman, M. Medical services at ultra-endurance foot races in remote environments: medical issues and consensus guidelines. Jentjens, R. Oxidation of combined ingestion of glucose and fructose during exercise. Jeukendrup, A. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. CrossRef Full Text Google Scholar. Lambert, G. Fluid tolerance while running: effect of repeated trials. Layer, P. Human pancreatic secretion and intestinal motility: effects of ileal nutrient perfusion. Liver Physiol. Lee, B. Whole body precooling attenuates the extracellular HSP72, IL-6 and IL responses after an acute bout of running in the heat. Lim, C. Heat sepsis precedes heat toxicity in the pathophysiology of heat stroke- a new paradigm on an ancient disease. Antioxidant McCubbin, A. Hydrogel carbohydrate electrolyte beverage does not improve blood glucose availability, carbohydrate malabsorption, gastrointestinal symptoms or carbohydrate oxidation during endurance running, or time to exhaustion performance test, compared to a standard concentration and nutrient-matched beverage. Sports Nutr. Miall, A. Two weeks of repetitive gut-challenge reduce exercise-associated GIS and malabsorption. Sports 2, — Möller, G. Supplementation of probiotics and its effects on physically active individuals and athletes: systematic review. Exerc Metab. Mujika, I. Case Study: Long-term low carbohydrate, high fat diet impairs performance and subjective wellbeing in a world-class vegetarian long-distance triathlete. Noakes, T. The importance of volume in regulating gastric emptying. Pasternak, A. Use of Ondansetron for nausea and vomiting during an ultra-endurance run. Sports Physiol. Péronnet, F. Table of nonprotein respiratory quotient: an update. Pfeiffer, B. Nutritional intake and gastrointestinal problems during competitive endurance events. Rauch, C. Non-protein oxidation rates of ultramarathon runners in response to incremental intensity and prolonged strenuous running. Rowlands, D. Lower oxidation of a high molecular weight glucose polymer vs. glucose during cycling. Multiple-Transportable Carbohydrate Effect on Long-Distance Triathlon Performance. No effect of protein coingestion on exogenous glucose oxidation during exercise. Russo, I. Assessing overall exercise recovery processes using carbohydrate and carbohydrate-protein containing recovery beverages. Does the nutritional composition of a dairy-based recovery beverage influence post-exercise gastrointestinal and immune status, and subsequent markers of recovery optimisation in response to high intensity interval exercise? Perform [Preprint]. Shin, H. Snipe, R. Does the temperature of water ingested during exertional-heat stress influence gastrointestinal injury, symptoms, and systemic inflammatory profile? Sport 21, — Does biological sex impact intestinal epithelial injury, small intestine permeability, gastrointestinal symptoms and systemic cytokine profile in response to exertional-heat stress? Heat stress during prolonged running results in exacerbated intestinal epithelial injury and gastrointestinal symptoms. Mild Heat stress during prolonged running results in exacerbated intestinal epithelial injury and gastrointestinal symptoms. Carbohydrate and protein intake during exertional-heat stress ameliorates intestinal epithelial injury and small intestine permeability. van Avesaat, M. Ileal brake activation: Macronutrient-specific effects on eating behavior? van Citters, G. Ileal brake: Neuropeptidergic control of intestinal transit. Warden, S. Zadow, E. Validity of power settings of the Wahoo KICKR power trainer. Keywords : circulatory-gastrointestinal, neuroendocrine-gastrointestinal, orocecal transit time, gastrointestinal symptoms, gastric motility, gastroparesis, ileus, intestinal epithelium. Citation: Gaskell SK, Rauch CE and Costa RJS Gastrointestinal Assessment and Therapeutic Intervention for the Management of Exercise-Associated Gastrointestinal Symptoms: A Case Series Translational and Professional Practice Approach. Received: 01 June ; Accepted: 05 August ; Published: 07 September Copyright © Gaskell, Rauch and Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. |
| 1. Introduction | Though much attention is centered around what to eat before running, what you eat afterward is equally important. Here are the 15 best foods to eat…. It also differs among…. Stretching before you run can help prevent injury. Learn about the most crucial muscle areas for runners, along with stretches to keep them healthy. If you're trying to increase your stamina while running, there are lots of things you can try. We've got 13 tips to get you running faster and longer. Training for a 5K is a fun way to get moving, and the type of plan you do will depend on your current fitness level, goals, and running experience. There may be some health benefits to running every day, but you may only need to run for 5 to 10 minutes a day. And running more than 4. Strengthening key muscles with these exercises will improve your running technique and help you avoid injury. Ideal running heart rates vary for each individual depending on several factors. Learn more about how to calculate the ideal heart rate for you. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Medically reviewed by Saurabh Sethi, M. What causes stomach problems during or after running? Was this helpful? How can you treat or prevent stomach problems during or after running? When to see a doctor. Key takeaways. Caregiver's responsibility would be to inform athletes about potential dangers of drinking too much water and also advise them to refrain from using hypertonic fluid replacements. The importance of physical activity to well-being cannot be overstated. The physiological, psychological, and social benefits of regular exercise are plentiful and profound. Examples of such benefits include positive effects on weight, bone strength, metabolic factors such as glucose and cholesterol , organ function, sleep, mood and self-image. Coupled with the proliferation of team sports and increased choices for individual exercise, the fitness movement has created an increased demand for the care of athletes. Anyone who participates in physical exercise is at risk for injury and illness arising from such activity [ 1 , 2 ]. Strenuous exercise and dehydrated states would be the causes of gastrointestinal symptoms. Gut ischemia would be the main cause of nausea, vomiting, abdominal pain and bloody diarrhea [ 3 ]. Moreover, anaphylaxis is observed during or soon after exercise when preceded by the intake of a causal food allergen [ 4 , 5 ]. Adequate meal composition and hydration are essential for the prevention of these events. There is a very high prevalence of gastrointestinal GI complaints during exercise among long-distance runners, triathletes and athletes involved in other types of strenuous long-lasting exercise [ 6 ]. These GI complaints occur because of the redistribution of the blood flow, that is shunted from the viscera to skeletal muscle, heart, lung and brain [ 7 ]. The symptoms include dizziness, nausea, stomach or intestinal clamps, vomiting and diarrhea. Severe symptoms include vomiting and diarrhea and occur mainly during running [ 8 ]. It has been suggested that these problems occur mainly because of the movements of the gut [ 9 ]. However, an association was reported between nutritional practices and GI complaints during a half ironman-distance triathlon with the intake of fiber, fat, protein and concentrated carbohydrate solutions during the triathlon, in particular beverages with very high osmolarity [ 10 ]. The symptoms are often mild and may not even affect performance. Some of the symptoms, however, can be life-threatening, such as blood loss in feces in the hours following the running presented by some marathoners and long-distance triathletes [ 8 ]. Damage to the gut and impaired gut function is associated with increased of intestinal permeability after a marathon [ 11 ]. Moreover, vigorous exercise jogging, aerobics, dancing, tennis, bicycling, racquetball, swimming, and skiing [ 12 , 13 ] facilities allergen absorption from the GI tract [ 14 ], leading to a food-dependent exercise induces anaphylaxis FDEIA. FDEIA is a subtype of anaphylaxis induced by exercise that is related to the intake of specific foods [ 15 ]. Allergic symptoms are elicited when triggering factors such as exercise or aspirin intake are added after intake of the causative food [ 16 ]. FDEIA is a unique disorder caused by exercise after food ingestion [ 17 ]. Ingestion of aspirin combined with exercise increased GI permeability in humans, thus allowing for the detection of food-derived allergens in serum [ 5 ]. When food intake and exercise are exposed independently, patients will not experience allergic symptoms [ 14 ]. However, the onset of anaphylaxis occurs during or soon after exercise when preceded by the ingestion of a causal food allergen [ 4 , 5 ]. FDEIA is an IgE-mediated hypersensitivity. As in other allergic syndromes, mast cells seem to play a prominent role, and most FDEIA symptoms can be explained based on the release of mast cell mediators, including histamine, leukotrienes LCT4 , and prostaglandins PGD2 [ 14 , 16 , 18 , 19 ]. Increased norepinephrine may be involved in the onset of FDEIA since it may selectively inhibit T-helper Th functions while favoring Th-2 responses [ 20 ]. Many kinds of food have been identified as causes of FDEIA, but any kind of food appears to be responsible for it. Specific FDEIA has been associated with cereals, seafood, peanut, free nuts, eggs, milk and vegetables [ 21 ]. FDEIA only occurs after consumption of a food allergen if this is followed by vigorous physical activity within a few hours of consumption [ 15 ]. Elicitation of the allergic symptoms is known to be dependent on the amount of the food intake [ 16 ]. FDEIA can be controlled by avoidance of food before exercise [ 13 ]. GI problems, hyperthermia and hyponatremia are potentially life-threatening in longer triathlon events. Problems with hyperthermia seem to be related to the intake of highly concentrated carbohydrate solutions, or hyperosmotic drinks, and the intake of fiber, fat and protein [ 8 ]. Hyponatremia has occasionally been reported, especially among slow competitors in triathlons, and probably arises from the loss of sodium in sweat in association with very high intake L of water or other low-sodium drinks [ 8 ]. During exercise, activity in the sympathoadrenal neuroendocrine system and its plasma hormones increases. Such increase is of major importance for cardiovascular adaptation, thermoregulation and energy-yielding substrate in exercise. Cardiac frequency and contraction force are enhanced; the tone of arterioles in the splanchnic area, kidney and non-contracting muscles and veins is increased, and the spleen is brought to contract. In this way, cardiac output is enhanced, and blood volume and flow are redistributed in favor of the skin and the working muscle [ 22 ]. Prolonged exercise at high intensities leads to a quantitative redistribution of blood flow to the exercising muscle exercise hyperthermia in proportion to its energy demands of oxygen and substrates. Sympathoadrenal activity, however, reduces water and sodium loss during exercise by decreasing renal blood flow and changing its distribution by direct tubular effects. Moreover, it decreases potassium loss by facilitating its muscular uptake [ 22 ]. Blood flow to the skin is increased to facilitate heat dissipation, and sweating implies loss of water and electrolytes from the body. The dehydrated state can be worsened by catecholamine-induced thirst suppression [ 24 ]. Fluid loss results in decreasing circulatory blood volume, blood pressure, sweat production and stroke volume, as result, vascular resistance increase leading to a skin blood flow decreased, all of which impair heat dissipation. Dehydration has a negative effect on endurance performance by increasing muscular glycogen degradation and plasma lactate levels and by causing cardiovascular drift and reduced ability to transport heat to the periphery for dissipation, thus resulting in increased core temperature [ 26 ]. Heat production during exercise is times greater than at rest, and it is sufficient to raise core body temperature by 1°C every 5 minutes if there are no thermoregulatory adjustments [ 25 ]. The body's multiple mechanisms for heat dissipation to prevent significant hyperthermia include conduction, convection, evaporation and radiation. As ambient temperature rises above 20°C, the contributions of conduction, convection and particularly radiation, become increasingly insignificant with the bulk of the heat dissipation during exercise resulting from evaporation as sweat. Sweat evaporation leads to dehydration, which increases body temperature [ 25 ]. Any factor that limits evaporation, such as high humidity or dehydration will have profound effects on physiological function, athletic performance, and risk for heat illness [ 27 ]. There are five common types of heat illness, the milder forms including heat edema, heat cramps, heat syncope, and heat exhaustion. The most severe form of heat illness is heat stroke [ 28 ]. The milder forms of heat illness are widely underreported and underdiagnosed [ 25 ]. The symptoms go from none to fatigue, mental confusion, nausea, and vomiting with the signs beginning with peripheral edema developing to muscular spasm, loss of consciousness, hypotension and elevated core temperature up to Heat stroke is defined as a condition in which body temperature is elevated to a level that causes damage to body tissues, giving rise to a characteristic clinical and pathological syndrome that affects multiple organs [ 29 ]. Distinguishing features of heat stroke are marked core body temperature elevations greater than Such decreased splanchnic blood flow and oxygen supply may induce changes in nutrient absorption, motility and the mucosal integrity of the GI tract, resulting in GI complaints [ 30 ]. The symptoms seem to occur more often during competition in a warm environment [ 30 ] in the presence of systemic dehydration and lower plasma volume [ 8 ]. The presence of dehydration in strenuous exercise in cyclists was shown to induce significantly increased nausea, epigastric cramps and delay in gastric emptying. Gastric emptying GE was significantly associated with increase in exercise-induced nausea. Exercise by itself led to significant increase in plasma vasopressin and rectal temperature and significant decrease in plasma volume, irrespective of the dehydration state, but vasopressin concentration was significantly higher in dehydrated athletes. By adding dehydration to strenuous cycling, there was a delayed gastric emptying, but no differences in orocecal transit time, intestinal permeability or glucose uptake [ 30 ]. In an endurance running experiment, GI complaints were reported only with the dehydration exercise combination without any GI disturbances being reported by athletes in either exercise or dehydration test alone. Dehydration-exercise resulted in slower GE than in other two treatments with the effects of dehydration and exercise being additives in delayed GE. Reduced GI blood flow induced by strenuous exercise makes the gut mucosa susceptible to ischemic injury, increases mucosa permeability and enhances hidden blood loss, as well as the translocation of protective microbiota and endotoxin generation. It is known that mucosal ischemia depletes cellular ATP leading to cell death and mucosal inflammation [ 11 , 38 ]. Poor conditioning, dietary factors and previous abdominal surgery are risk factors with weak evidence that was not well supported [ 39 ]. Rapid fluid delivery from beverages intake is the goal of oral rehydration solutions and sports drinks [ 40 ]. The goal of fluid intake is to consume more fluid orally than it is being lost in sweat. Extracellular fluid rehydration is best achieved with smaller fluid volumes and isotonic sodium solutions. Intracellular rehydration is best achieved with higher volumes and lower sodium hypotonic solutions. The addition of sodium and carbohydrates assists with intestinal absorption of water and permits more efficient fluid replacement than water alone [ 2 ]. The maximum rate of intestinal absorption is 0. It was estimated that ~ 0. The intake of large volumes may not be advantageous [ 8 ], because no enhance in performance is observed [ 41 , 42 ]. Fluid delivery during exercise represents the integration of GE and intestinal absorption. GE of liquids is regulated by the interaction of gastric volume and feedback inhibition, including nutrient-induced duodenal feedback. This occurs in such a way that the GE rate at any given moment represents the balance between feedback inhibition from the small intestine and the stimulatory effect of gastric volume. It is this balance that is responsible for the inverse relationship between beverage CHO content and GE rate [ 43 ]. Fluids empty from the stomach in an exponential manner with an initial rapid emptying phase. In fact, one of the major stimulants of GE is the volume in the stomach with a positive relationship between stomach volume and rate of emptying from the stomach. The absorption of water in the intestine is primarily passive, where water passes across the intestinal membrane due to an osmotic gradient [ 8 ]. Fluid absorption was given during 85 min of cycling exercise There were no differences between groups in GE, total fluid absorption, urine production or plasma volume variations. Water was absorbed faster from the duodenum than the jejunum. The effectiveness of different carbohydrate solutions in restoring fluid balance in situations of voluntary fluid intake was examined in 1. Beginning 30 min after cessation of exercise, the subjects drank ad libitum for a period of minutes. No differences were observed in total fluid intake, urine output, net fluid balance or in the fraction of the drink intake retained. The authors concluded that in situations of voluntary fluid intake, hypertonic carbohydrate-electrolyte solutions are as effective as hypotonic carbohydrate-electrolyte solutions at restoring whole-body fluid balance [ 26 ]. Glucose is actively transported across the intestinal membrane, a process aided by the inclusion of sodium. Water co-transportation during this process is controversial; nevertheless, the addition of sodium and CHO to sports drinks is widely recommended to enhance water absorption [ 8 ]. The risks of exercise-induced fluid and electrolyte balance are considerably minimized if oral replacement products are used. If activity is prolonged beyond 60 minutes, then CHO sources and potassium should also be included in the ingested fluid [ 2 ]. There is convincing evidence that the limitation of 1. Intestinal perfusion studies suggest that the capacity to absorb glucose is only slightly in excess of the observed entrance of glucose into the blood, and the absorption rate may thus be a factor that contributes to the limitations. The liver, however, may play an additional important role in that it provides glucose to the blood stream at a rate of only 1. It is possible that when large amounts of glucose are ingested, absorption is a limiting factor, and the liver will retain some glucose and will thus act as a second limiting factor to exogenous CHO oxidation [ 8 ]. Furthermore, the intake of a glucose-fructose combined solution increases GE and fluid delivery when compared with a glucose-only solution. Additionally, the combined sugar attenuates heart-rate increase and results in lower rates of perceived exertion and lower loss of body weight than glucose alone or water [ 43 ]. Moreover, a solution intake with 1. Hypertonic solutions tend to delay water absorption in the intestine as water instead flows into the intestine to dilute the solution before water is absorbed [ 8 ]. Additionally, there is contention as to whether hypertonic solutions reduce the GE rate [ 46 ]. Exercise regulation of intestinal tight junction proteins. Br J Sports Med. Gaskell SK, Rauch CE, Parr A, Costa RJS. Diurnal versus nocturnal exercise-effect on the gastrointestinal tract. Costa RJS, Camões-Costa V, Snipe RMJ, Dixon D, Russo I, Huschtscha Z. Impact of exercise-induced hypohydration on gastrointestinal integrity, function, symptoms, and systemic endotoxin and inflammatory profile. Gaskell SK, Snipe RMJ, Costa RJS. Test-Retest reliability of a modified visual analog scale assessment tool for determining incidence and severity of gastrointestinal symptoms in response to exercise stress. Int J Sport Nutr Exerc Med. Horner KM, Schubert MM, Desbrow B, Byrne NM, King NA. Acute exercise and gastric emptying: a meta-analysis and implications for appetite control. Leiper JB. Fate of ingested fluids: factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr Rev. Nolan JD, Johnston IM, Walters JRF. Physiology of malabsorption. Bate JP, Irving PM, Barrett JS, Gibson PR. Benefits of breath hydrogen testing after lactulose administration in analysing carbohydrate malabsorption. Eur J Gastroenterol and Hepatol. Lindberg G, Iwarzon M, Hammarlund B. Maurer AH. Gastrointestinal motility, part 1: esophageal transit and gastric emptying. J Nucl Med. Kalantar-Zadeh K, Berean KJ, Ha N, Chrimes AF, Xu K, Grando D, et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat Electron. Noakes TD, Rehrer NJ, Maughan RJ. The importance of volume in regulating gastric emptying. Born P. Carbohydrate malabsorption in patients with non-specific abdominal complaints. World J Gastroenterol. Yao CK, Tuck CJ, Barrett JS, Canale KE, Philpott HL, Gibson PR. Poor reproducibility of breath hydrogen testing: Implications for its application in functional bowel disorders. United European Gastroenterol J. Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta - Biomembr. Costa RJ, Gill S, Snipe R, Gaskell S, Russo I, Burke L. Assessment of exercise-associated gastrointestinal perturbations in research and practical settings: Methodological concerns and recommendations for best practice. Int J Sport Nutr Exerc Metab in press. Peake JM, della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. PubMed Google Scholar. Suzuki K, Tominaga T, Ruhee RT, Ma S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Cabral-Santos C, de Lima Junior EA, da Fernandes IMC, Pinto RZ, Rosa-Neto JC, Bishop NC, et al. Interleukin responses from acute exercise in healthy subjects: a systematic review. J Cell Physiol. Gill SK, Hankey J, Wright A, Marczak S, Hemming K, Allerton DM, et al. The impact of a h ultra-marathon on circulatory endotoxin and cytokine profile. Gill SK, Teixeira A, Rama L, Prestes J, Rosado F, Hankey J, et al. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, et al. Carbohydrate and the cytokine response to 2. Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, et al. Influence of mode and carbohydrate on the cytokine response. Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol. Bishop NC, Walsh NP, Haines DL, Richards EE, Gleeson M. Pre-exercise carbohydrate status and immune responses to prolonged cycling: II. Effect on plasma cytokine concentration. Arribalzaga S, Viribay A, Calleja-González J, Fernández-Lázaro D, Castañeda-Babarro A, Mielgo-Ayuso J. Relationship of carbohydrate intake during a single-stage one-day ultra-trail race with fatigue outcomes and gastrointestinal problems: a systematic review. Int J Environ Res. Costa RJS, Teixeira A, Rama L, Swancott AJM, Hardy LD, Lee B, et al. Water and sodium intake habits and status of ultra-endurance runners during a multi-stage ultra-marathon conducted in a hot ambient environment: an observational field based study. Nutr J. Wilson PB, Rhodes GS, Ingraham SJ. Saccharide Composition of Carbohydrates Consumed during an Ultra-endurance Triathlon. J Am Coll Nutr. Drossman DA. The Functional Gastrointestinal Disorders and the Rome III Process. Peters HPF, Bos M, Seebregts L, Akkermans LMA, van Berge Henegouwen GP, Bol E, et al. Gastrointestinal symptoms in long-distance runners, cyclists, and triathletes: prevalence, medication, and etiology. Am J Gastroenterol. Rehrer NJ, Meijer GA. Biomechanical vibration of the abdominal region during running and bicycling. J Sports Med Phys Fitness. CAS PubMed Google Scholar. More than a gut feeling: What is the role of the gastrointestinal tract in female athlete health? Eur J Sport Sci. Riddoch C, Trinick T. Gastrointestinal disturbances in marathon runners. Hoffman M. Considerations for ultra-endurance activities: part 2-hydration. Res Sports Med. Killian LA, Lee SY. Irritable bowel syndrome is underdiagnosed and ineffectively managed among endurance athletes. Bengtsson M, Persson J, Sjölund K, Ohlsson B. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol Nurs. Goodman BE. Insights into digestion and absorption of major nutrients in humans. Adv Physiol Educ. Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev. Peters HPF, Schep G, Koster DJ, Douwes AC, de Vries WR. Hydrogen breath test as a simple noninvasive method for evaluation of carbohydrate malabsorption during exercise. Eur J Appl Physiol Occup Physiol. Snipe RMJ, Costa RJS. Does biological sex impact intestinal epithelial injury, small intestine permeability, gastrointestinal symptoms and systemic cytokine profile in response to exertional-heat stress? Download references. Department of Nutrition, Dietetics and Food, Monash University, Level 1, Ferntree Gully Road, Notting Hill, VIC, , Australia. Isabel G. Martinez, Alice S. Mika, Jessica R. You can also search for this author in PubMed Google Scholar. Correspondence to Ricardo J. Isabel Martinez, Alice Mika, Jessica Biesiekierski, and Ricardo Costa declare that they have no conflicts of interest relevant to the content of this review. Listed authors were all involved in the SLR process and preparation and review of the manuscript. Search strategy was designed by IM, JB, and RC. Studies were screened and selected by IM, AM, and JB. IM and AM extracted the data and conducted risk-of-bias assessments. The first draft of the manuscript was written by IM and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Martinez, I. et al. The Effect of Gut-Training and Feeding-Challenge on Markers of Gastrointestinal Status in Response to Endurance Exercise: A Systematic Literature Review. Sports Med 53 , — Download citation. Accepted : 13 March Published : 15 April Issue Date : June Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract Background Nutrition during exercise is vital in sustaining prolonged activity and enhancing athletic performance; however, exercise-induced gastrointestinal syndrome EIGS and exercise-associated gastrointestinal symptoms Ex-GIS are common issues among endurance athletes. Results Overall, studies were identified, and eight studies were included after screening. Conclusions Overall, gut-training or feeding-challenge around exercise may provide advantages in reducing gut discomfort, and potentially improve carbohydrate malabsorption and Ex-GIS, which may have exercise performance implications. Post-exercise Ingestion of Carbohydrate, Protein and Water: A Systematic Review and Meta-analysis for Effects on Subsequent Athletic Performance Article 02 November Training the Gut for Athletes Article Open access 22 March Effect of Glycemic Index of a Pre-exercise Meal on Endurance Exercise Performance: A Systematic Review and Meta-analysis Article 28 September Use our pre-submission checklist Avoid common mistakes on your manuscript. FormalPara Key Points Repetitive exposure to nutrition before and during exercise can help train the gastrointestinal tract, and subsequently improve gastrointestinal function, feeding tolerance, and reduce incidence and severity of exercise-associated gastrointestinal symptoms Ex-GIS , leading to potential exercise performance benefits. Full size image. Table 1 Participant Intervention Comparator Outcomes Study PICOS criteria for study eligibility Full size table. Table 2 General characteristics of included studies and gut-training or feeding-challenge protocol Full size table. Table 3 Effect of gut-training or feeding-challenges on gastrointestinal integrity in response to exercise Full size table. Table 4 Effect of gut-training or feeding-challenges on gastrointestinal functional responses in response to exercise Full size table. Table 5 Effect of gut-training or feeding-challenges on systemic immune response to exercise Full size table. Table 6 Effect of gut-training or feeding-challenges on gastrointestinal symptoms in response to exercise Full size table. Table 8 Risk of bias assessment results Full size table. References Burke LM, Castell LM, Casa DJ, Close GL, Costa RJS, Melin AK, et al. Article CAS PubMed Google Scholar Thomas DT, Erdman KA, Burke LM. Article PubMed Google Scholar Costa RJS, Knechtle B, Tarnopolsky M, Hoffman MD. Article CAS PubMed Google Scholar Costa RJS, Hoffman MD, Stellingwerff T. Article CAS PubMed Google Scholar Gaskell SK, Rauch CE, Costa RJS. Article Google Scholar Stellingwerff T, Cox GR. Article PubMed Google Scholar Snipe RMJ, Khoo A, Kitic CM, Gibson PR, Costa RJS. Article CAS PubMed Google Scholar ter Steege RWF, van der Palen J, Kolkman JJ. Article Google Scholar Costa RJS, Gill SK, Hankey J, Wright A, Marczak S. Article CAS PubMed Google Scholar Costa RJS, Swancott AJ, Gill S, Hankey J, Scheer V, Murray A, et al. Google Scholar Geesmann B, Mester J, Koehler K. Article PubMed Google Scholar Pfeiffer B, Stellingwerff T, Hodgson AB, Randell R, Pöttgen K, Res P, et al. Article CAS PubMed Google Scholar Barrero A, Erola P, Bescós R. Article PubMed PubMed Central Google Scholar Costa RJS, Snipe R, Camões-Costa V, Scheer V, Murray A. Article PubMed PubMed Central Google Scholar Costa RJS, Snipe RMJ, Kitic CM, Gibson PR. Article CAS PubMed Google Scholar Rauch CE, McCubbin AJ, Gaskell SK, Costa RJS. Article Google Scholar Muros JJ, Sánchez-Muñoz C, Hoyos J, Zabala M. Article PubMed Google Scholar Rehrer NJ, Hellemans IJ, Rolleston AK, Rush E, Miller BF. Article CAS PubMed Google Scholar Costa R, Gaskell SK, McCubbin AJ, Snipe RMJ. Article Google Scholar Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Article CAS PubMed Google Scholar Lang JA, Gisolfi CV, Lambert GP. Article CAS PubMed Google Scholar Snipe RMJ, Khoo A, Kitic CM, Gibson PR, Costa RJS. Article CAS Google Scholar Hearris MA, Pugh JN, Langan-Evans C, Mann SJ, Burke L, Stellingwerff T, et al. Article CAS PubMed Google Scholar King AJ, Etxebarria N, Ross ML, Garvican-Lewis L, Heikura IA, Mckay AKA, et al. Article CAS PubMed PubMed Central Google Scholar Rehrer NJ, Browns F, Beckers EJ, Hoor F, Saris WHM. Article CAS PubMed Google Scholar Jeukendrup AE. Article PubMed PubMed Central Google Scholar Jeukendrup AE, McLaughlin J. Article CAS PubMed Google Scholar Murray R. Article PubMed Google Scholar Dyer J, Daly K, Salmon KSH, Arora DK, Kokrashvili Z, Margolskee RF, et al. Article CAS PubMed Google Scholar Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, et al. Article CAS PubMed PubMed Central Google Scholar Miyamoto K, Hase K, Tagagi T, Fuji T, Taketani Y, Minami H, et al. Article CAS PubMed PubMed Central Google Scholar Cunningham KM, Horowitz M, Read NW. Article CAS PubMed Google Scholar Horowitz M, Cunningham KM, Wishart JM, Jones KL, Read NW. Article CAS PubMed Google Scholar Lin HC, Doty JE, Reedy TJ, Meyer JH. Article PubMed Google Scholar Shin HS, Ingram JR, McGill AT, Poppitt SD. Article CAS PubMed Google Scholar Minami H, McCallum RW. Article CAS PubMed Google Scholar Cox GR, Clark SA, Cox AJ, Halson SL, Hargreaves M, Hawley JA, et al. Article CAS PubMed Google Scholar Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Article CAS PubMed Google Scholar Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. Article Google Scholar Veritas Health Innovation. Article PubMed Google Scholar Houmard JA, Egan PC, Johns RA, Neufer PD, Chenier TC, Israel RG. Article CAS PubMed Google Scholar Miall A, Khoo A, Rauch C, Snipe RMJ, Camões-Costa VL, Gibson PR, et al. Article CAS PubMed Google Scholar Cox AJ, Pyne DB, Cox GR, Callister R, Gleeson M. Article CAS PubMed Google Scholar Svendsen IS, Killer SC, Carter JM, Randell RK, Jeukendrup AE, Gleeson M. Article CAS PubMed PubMed Central Google Scholar van Wijck K, Lenaerts K, Grootjans J, Wijnands KAP, Poeze M, van Loon LJC, et al. Article CAS Google Scholar ter Steege RWF, Kolkman JJ. Article PubMed Google Scholar Evennett NJ, Petrov MS, Mittal A, Windsor JA. Article PubMed Google Scholar Ricciuto A, Griffiths AM. Article CAS PubMed Google Scholar Garcia-Hernandez V, Quiros M, Nusrat A. Article CAS PubMed PubMed Central Google Scholar Lambert P. Article PubMed Google Scholar Kaparakis-Liaskos M, Ferrero RL. Article CAS PubMed Google Scholar Camus G, Poortmans J, Nys M, Deby-Dupont G, Duchateau J, Deby C, et al. Article CAS Google Scholar Bosenberg AT, Brock-Utne JG, Gaffin SL, Wells MTB, Blake GTW. Article CAS PubMed Google Scholar Stuempfle KJ, Hoffman MD. Article PubMed Google Scholar Young P, Rauch C, Russo I, Gaskell S, Davidson Z, Costa RJS. Article PubMed PubMed Central Google Scholar van Wijck K, Lenaerts K, van Loon LJC, Peters WHM, Buurman WA, Dejong CHC. Article PubMed PubMed Central Google Scholar March DS, Marchbank T, Playford RJ, Jones AW, Thatcher R, Davison G. Article CAS Google Scholar Costa R, Young P, Gill SK, Snipe RMJ, Gaskell S, Russo I, et al. Article PubMed Google Scholar Matheson PJ, Wilson MA, Garrison RN. Article CAS PubMed Google Scholar Rehrer NJ, Goes E, DuGardeyn C, Reynaert H, DeMeirleir K. Article CAS PubMed Google Scholar Zuhl M, Schneider S, Lanphere K, Conn C, Dokladny K, Moseley P. Article PubMed Google Scholar Gaskell SK, Rauch CE, Parr A, Costa RJS. Article CAS PubMed Google Scholar Costa RJS, Camões-Costa V, Snipe RMJ, Dixon D, Russo I, Huschtscha Z. Article CAS PubMed Google Scholar Gaskell SK, Snipe RMJ, Costa RJS. Article Google Scholar Horner KM, Schubert MM, Desbrow B, Byrne NM, King NA. Article PubMed Google Scholar Leiper JB. Article PubMed Google Scholar Nolan JD, Johnston IM, Walters JRF. Article Google Scholar Bate JP, Irving PM, Barrett JS, Gibson PR. Article CAS Google Scholar Lindberg G, Iwarzon M, Hammarlund B. Article CAS PubMed Google Scholar Maurer AH. Article CAS PubMed Google Scholar Kalantar-Zadeh K, Berean KJ, Ha N, Chrimes AF, Xu K, Grando D, et al. Article Google Scholar Noakes TD, Rehrer NJ, Maughan RJ. Article CAS PubMed Google Scholar Born P. Article CAS PubMed PubMed Central Google Scholar Yao CK, Tuck CJ, Barrett JS, Canale KE, Philpott HL, Gibson PR. Article CAS PubMed Google Scholar Capaldo CT, Nusrat A. Article CAS Google Scholar Costa RJ, Gill S, Snipe R, Gaskell S, Russo I, Burke L. PubMed Google Scholar Suzuki K, Tominaga T, Ruhee RT, Ma S. Article PubMed PubMed Central Google Scholar Cabral-Santos C, de Lima Junior EA, da Fernandes IMC, Pinto RZ, Rosa-Neto JC, Bishop NC, et al. Article CAS PubMed Google Scholar Gill SK, Hankey J, Wright A, Marczak S, Hemming K, Allerton DM, et al. Article CAS PubMed Google Scholar Gill SK, Teixeira A, Rama L, Prestes J, Rosado F, Hankey J, et al. PubMed Google Scholar Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, et al. |
Welche nötige Wörter... Toll, der bemerkenswerte Gedanke
Ich entschuldige mich, aber meiner Meinung nach lassen Sie den Fehler zu. Ich kann die Position verteidigen.
JA, die Variante gut