Amino acid catabolism -
Only glucogenic aa do. Ariel Tan. The liver uses AA for protein synthesis, or sends it to other tissues in the body for protein synthesis.
Does this mean only "excess" AA is used for metabolism? The body will preferentially use AA for protein synthesis unless there's a significant surplus?
Or is the body always using a little bit for energy? What triggers AA metabolism then? How does the body know when AAs are "in excess"? Posted 10 months ago. Could Khan make a video specifically about the Urea Cycle? I've been using Khan for Biochemistry. Candace Lei. Do we have to memorize the process of generation of ketone bodies from keto acid for MCAT?
Julian Burton-Pierce. At Video transcript - [Instructor] In this video, I wanna provide you with a crash course overview of amino acid metabolism. And, specifically, I wanna focus on the catabolism of amino acids and how that catabolism allows us to produce ATP inside of ourselves.
Now, compared to carbohydrate catabolism and fatty acid catabolism, recall the pathways of glycolysis and fatty acid oxidation. So that's why I think that amino acid metabolism doesn't usually get its fair share of airtime, compared to processes like glycolysis and fatty acid oxidation.
And to do that, let's go ahead and follow what happens to amino acids in the fed, as well as the fasted states of our body. Now, fed refers to our body's state right after, immediately after eating a meal. And, remember, that in terms of hormones, the hormone that's going to be elevated is going to be insulin, which is elevated in response to higher blood glucose levels, immediately following a meal, and levels of the hormone glucagon are going to be decreased.
Now, of course, this is going to be opposite several hours after a meal, which we called the fasted state, in which the levels of insulin will be decreased and, of course, in response to low blood glucose levels, the levels of glucagon in our body will start to rise along with a couple of other hormones as well.
But these are the two, or two at least, big hormones that regulate the bulk of metabolism in our body. Now, starting with the fed state, let's start at the beginning of this story. Recall that we ingest proteins from our food and those proteins are broken down into amino acids inside of our small intestine.
And just as a side note, you might hear the terms essential and non-essential amino acids used, especially in medical literature. And what this simply refers to is that essential amino acids are those amino acids, of the 20 that we know of, that our body cannot synthesize and so we must, somehow, get these in our diet.
Whereas non-essential amino acids can be actually synthesized in our body and we don't need them as part of our diet. But, getting back to these amino acids, once they're broken down in the small intestine, they travel via the blood stream directly to the liver, just like glucose.
Now, once the amino acids have made it to the liver, several things can happen. The liver can use these amino acids directly for protein synthesis.
And, of course, recall that the storage, the ultimate storage forms of these two molecules are gonna be glycogen, in the case of glucose, which is stored in the liver mainly, and, for fatty acids, we store these as triacylglycerides in our adipose tissue.
So how did this conversion from amino acids to glucose and fatty acids happen, you might ask? Well, remember that the precursor for glucose, or I should say precursors, can be pyruvate as well as oxaloacetate.
And, for fatty acids, the main precursor for fatty acid synthesis is the molecule acetyl-CoA. And, as a relevant side note, I wanna point out that acetyl-CoA happens to be in equilibrium with another molecule in the cell called acetoacetyl-CoA.
And oxaloacetate if you remember is in equilibrium with a lot of the intermediates of the Krebs cycle. So I'm gonna abbreviate here as intermediates of Krebs cycle, and there are numerous molecules with numerous names that I won't mention here, but just so that you get the big picture.
Now the key point here is that amino acids, specifically the carbon backbone of these amino acid molecules can be interconverted and metabolized directly into the molecules in the precursor molecules that I've listed here for fatty acids and glucose.
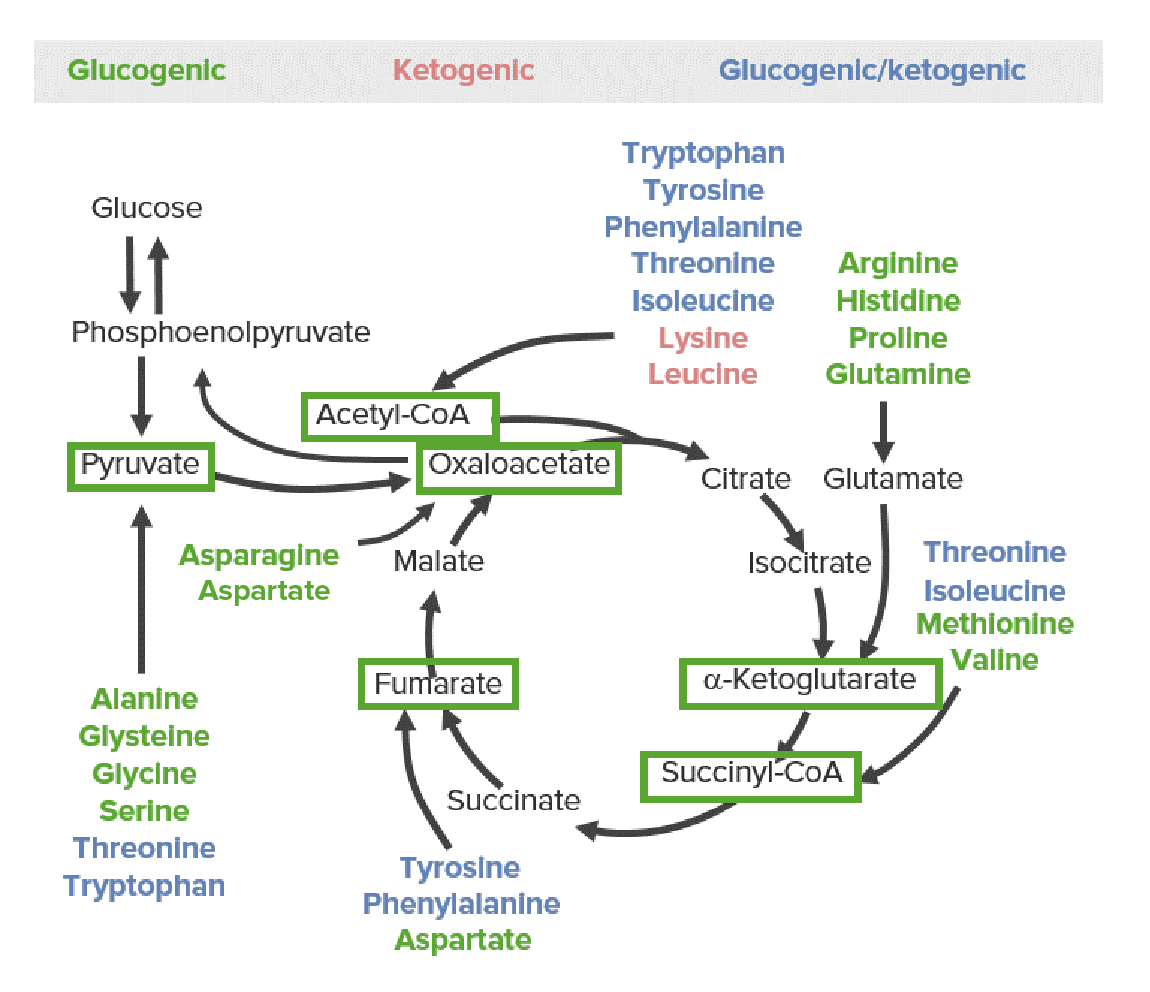
So they can be converted directly into pyruvate, into oxaloacetate, as well as intermediates of the Krebs cycle, acetyl-CoA, as well as acetoacetyl-CoA. Now another classification that you might hear with regard to amino acids is whether an amino acid is so-called a ketogenic amino acid or whether it is a glucogenic amino acid, and that simply refers to whether the carbon backbone of these amino acid molecules feeds into the precursor molecules for glucose synthesis or whether it feeds into the precursor molecules for fatty acid synthesis.
So in this case, ketogenic amino acids are converted to acetyl-CoA or acetoacetyl-CoA and ultimately fatty acids, whereas glucogenic amino acids feed into pyruvate, oxaloacetate, or intermediates of the Krebs cycle.
Now just as a fun fact, it turns out that there are two amino acids that are exclusively ketogenic and those are lysine and leucine. So anytime you ingest lysine or leucine, you will definitely be making fatty acids from those amino acids if they're ingested in excess.
Plant Cell 25, — Armbrust, E. The genome of the diatom Thalassiosira pseudonana : ecology, evolution, and metabolism. Science , 79— Bauwe, H. Genetic manipulation of glycine decarboxylation. Bender, S.
Transcriptional responses of three model diatoms to nitrate limitation of growth. Bowler, C. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Bromke, M. Amino acid biosynthesis pathways in diatoms. Metabolites 3, — Butler, T.
Phaeodactylum tricornutum : a diatom cell factory. Trends Biotechnol. Campbell, M. Genes encoding for branched-chain amino acid aminotransferase are differentially expressed in plants. Plant Physiol. Contreras, J. Asparagine-based production of hydrogen peroxide triggers cell death in the diatom Phaeodactylum tricornutum.
Dixon, D. Enzymes of tyrosine catabolism in Arabidopsis thaliana. Plant Sci. Fabris, M. The metabolic blueprint of Phaeodactylum tricornutum reveals a eukaryotic Entner—Doudoroff glycolytic pathway. Plant J. Falciatore, A. Diatom molecular research comes of age: model species for studying phytoplankton biology and diversity.
Plant Cell 32, — Forde, B. Glutamate in plants: metabolism, regulation, and signalling. Ge, F. Methylcrotonyl-CoA carboxylase regulates triacylglycerol accumulation in the model diatom Phaeodactylum tricornutum. Plant Cell 26, — Geider, R.
Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Guerra, L. Regulatory branch points affecting protein and lipid biosynthesis in the diatom Phaeodactylum tricornutum. Biomass Bioenerg. Hasse, D. Alternative splicing produces an H-protein with better substrate properties for the P-protein of glycine decarboxylase.
FEBS J. Häusler, R. Amino acids — A life between metabolism and signaling. Hildebrandt, T. Amino acid catabolism in plants. Plant 8, — Hockin, N. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants.
Ireland, R. Biochemistry and Biotechnology , ed. Singh New York, NY: Marcel Dekker , 49— Google Scholar. Jauffrais, T. Physiological and photophysiological responses of the benthic diatom Entomoneis paludosa Bacillariophyceae to dissolved inorganic and organic nitrogen in culture.
Karas, B. Designer diatom episomes delivered by bacterial conjugation. Kudela, R. Nutrient regulation of phytoplankton productivity in Monterey Bay, California. Deep Sea Res. Part II Top. Lancien, M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation.
Levering, J. Genome-scale model reveals metabolic basis of biomass partitioning in a model diatom. PLoS One e Integrated regulatory and metabolic networks of the marine diatom Phaeodactylum tricornutum predict the response to rising CO2 levels.
mSystems 2:e Levitan, O. Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress. Liang, Y. Branched-chain amino acid catabolism impacts triacylglycerol homeostasis in Chlamydomonas reinhardtii. Litwack, G. Matthijs, M. Profiling of the early nitrogen stress response in the diatom Phaeodactylum tricornutum reveals a novel family of RING-domain transcription factors.
The transcription factor bZIP14 regulates the TCA cycle in the diatom Phaeodactylum tricornutum. EMBO J. Mazelis, M. Miflin Cambridge, MA: Academic Press , — Medlin, L. Witkowski and J. Sieminska Krakow: Polish Academy of Sciences , 13— Moore, C. Processes and patterns of oceanic nutrient limitation.
Morris, S. Regulation of enzymes of the urea cycle and arginine metabolism. Moustafa, A. Genomic footprints of a cryptic plastid endosymbiosis in diatoms.
Science , — Oliveira, I. Molecular-genetic dissection of ammonium assimilation in Arabidopsis thaliana. Palenik, B. Comparison of cell-surface L-amino acid oxidases from several marine phytoplankton. Pan, Y. B Biol. Polekhina, G. Biochemistry 40, — Rébeillé, F.
Methionine catabolism in Arabidopsis cells is initiated by a γ-cleavage process and leads to S-methylcysteine and isoleucine syntheses.
Rees, T. Evidence for an extracellular L—amino acid oxidase in nitrogen-deprived Phaeodactylum tricornutum Bacillariophyceae and inhibition of enzyme activity by dissolved inorganic carbon.
Phycologia 45, — Reitzer, L. Cellular and Molecular Biology , Vol. Neidhardt, J. Ingraham, K. Low, B. Magasanik, M. Schaechter, and H. Umbarger Washington, DC: American Society for Microbiology , — Remmers, I. Orchestration of transcriptome, proteome and metabolome in the diatom Phaeodactylum tricornutum during nitrogen limitation.
Algal Res. Ros, R. The latter alternative, amino acid catabolism, is more likely to occur when glucose levels are low—for example, when a person is fasting or starving.
Transamination is an exchange of functional groups between any amino acid except lysine, proline, and threonine and an α-keto acid. Transamination reactions are catalyzed by specific transaminases also called aminotransferases , which require pyridoxal phosphate as a coenzyme.
This reaction occurs primarily in liver mitochondria. The synthesis of glutamate occurs in animal cells by reversing the reaction catalyzed by glutamate dehydrogenase.
Branched-chain catbaolism acids BCAAs are Amino acid catabolism catabopism skeletal muscle and whole-body Amkno and energy homeostasis. Amino acid catabolism has implication for Energy monitoring tools metabolism. However, elevated circulating levels of BCAAs and of their ketoacids as well as impaired Qcid of these amino acids Catabbolism are implicated in Amino acid catabolism development of insulin resistance Amino acid catabolism its sequelae, including type 2 diabetes, cardiovascular disease, and of some cancers, although other studies indicate supplements of these AAs may help in the management of some chronic diseases. Here, we first reviewed the catabolism of these AAs especially in skeletal muscle as this tissue contributes the most to whole body disposal of the BCAA. We then reviewed emerging mechanisms of control of enzymes involved in regulating BCAA catabolism. Such mechanisms include regulation of their abundance by microRNA and by post translational modifications such as phosphorylation, acetylation, and ubiquitination. We also reviewed implications of impaired metabolism of BCAA for muscle and whole-body metabolism. In molecular Amino acid catabolismprotein catabolism is the breakdown of qcid Amino acid catabolism Water weight reduction tips peptides catabolksm ultimately into amino acids. Protein catabolism is a key function of digestion process. Protein catabolism often begins with pepsinwhich converts proteins into polypeptides. These polypeptides are then further degraded. In humans, the pancreatic proteases include trypsinchymotrypsinand other enzymes.
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Schreiben Sie mir in PM, wir werden besprechen.
Welche Wörter... Die Phantastik