Chitosan for wound dressing -
China E-mail: heliu ciac. cn , cathwang hotmail. com , evanlee com , qinyanguo hotmail. com , wangzhjlu outlook. com , qq. com , lizuhao com , jinchengwang hotmail. b Hallym University, 1Hallymdaehak-gil, Chuncheon, Gangwon-do, Korea. Functional active wound dressings are expected to provide a moist wound environment, offer protection from secondary infections, remove wound exudate and accelerate tissue regeneration, as well as to improve the efficiency of wound healing.
Chitosan-based hydrogels are considered as ideal materials for enhancing wound healing owing to their biodegradable, biocompatible, non-toxic, antimicrobial, biologically adhesive, biological activity and hemostatic effects. Chitosan-based hydrogels have been demonstrated to promote wound healing at different wound healing stages, and also can alleviate the factors against wound healing such as excessive inflammatory and chronic wound infection.
The unique biological properties of a chitosan-based hydrogel enable it to serve as both a wound dressing and as a drug delivery system DDS to deliver antibacterial agents, growth factors, stem cells and so on, which could further accelerate wound healing.
For various kinds of wounds, chitosan-based hydrogels are able to promote the effectiveness of wound healing by modifying or combining with other polymers, and carrying different types of active substances.
In this review, we will take a close look at the application of chitosan-based hydrogels in wound dressings and DDS to enhance wound healing. Liu, C. Wang, C. Li, Y. Qin, Z. Wang, F. Yang, Z. Hesperidin has been reported to possess the capability of repairing deep wounds through inducing VEGF gene expression and stimulating the growth of epithelial cells and collagen deposition Haddadi et al.
It was loaded into alginate-chitosan hydrogels with various concentrations to enhance deep wound healing. The in vivo tests verified that wound closure was accelerated compared to treatment with bandages Bagher et al. The modification of hydrogels with cells and bioactive molecules like growth factors has drawn attention due to its ability to accelerate wound healing by inducing intracellular signaling and stimulating the synthesis of skin repair-related proteins Guo et al.
Adipose-derived stem cells ASCs can secrete several types of angiogenic growth factors that promote angiogenesis in wounded tissue Guilak et al. Cheng et al. In vitro tests showed that a higher concentration of VEGF was present in the supernatant of the hydrogel.
This hydrogel can be used for therapeutic angiogenesis. BMSCs have been shown to be a candidate for cell therapy for keloids, a kind of benign fibroproliferative tumor in which dermal and subcutaneous extracellular matrices excessively accumulate and outgrow the original wounded area Fang et al.
A keloid is not only unaesthetic but also prone to becoming cancerous. A previous study reported that Arg-Gly-Asp-grafted hydroxybutyl chitosan hydrogel combined with BMSCs inhibited extracellular matrix synthesis via paracrine signaling, and thus, is a promising candidate for subcutaneous injection treatment for keloids Qu et al.
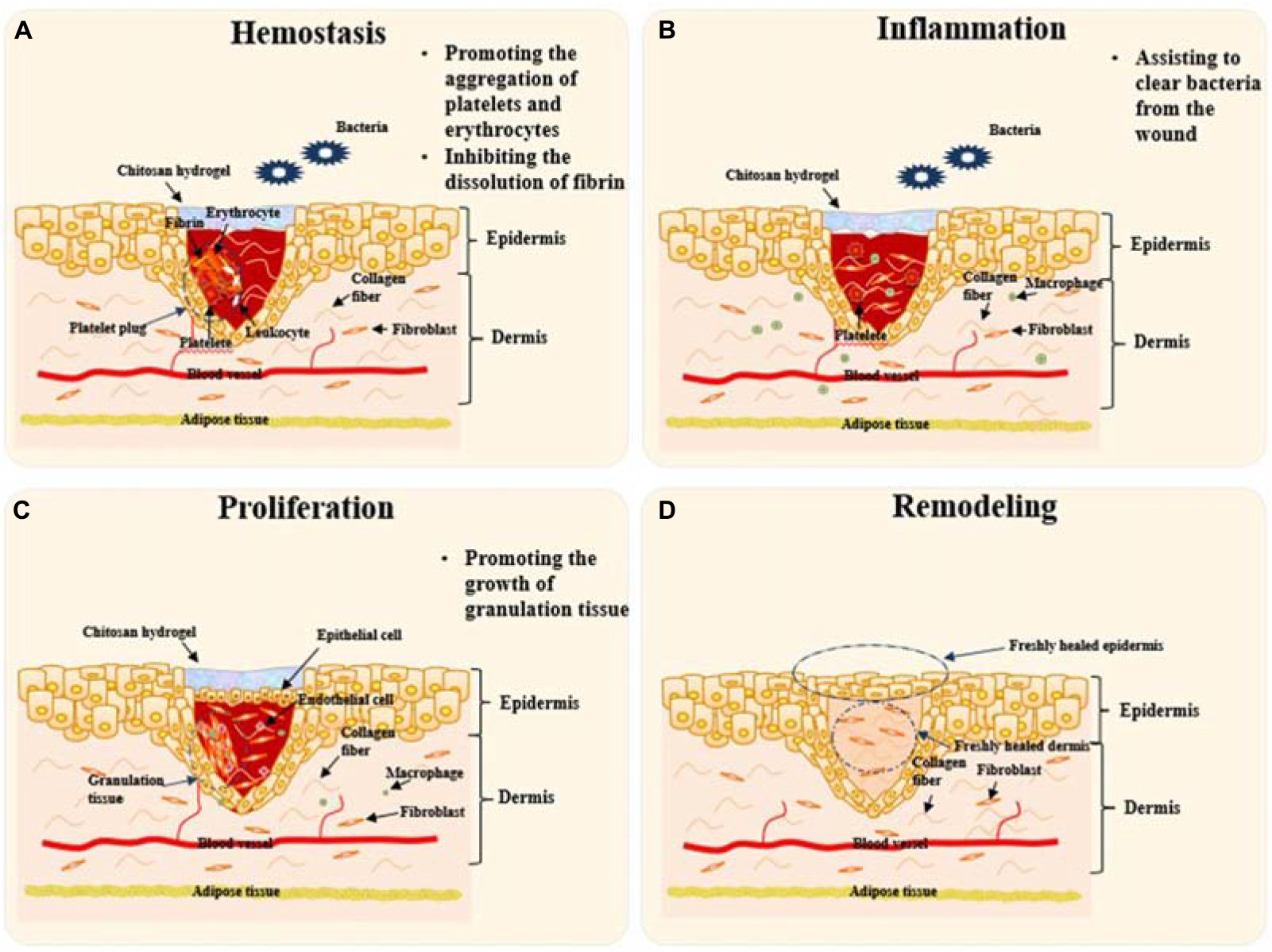
A variety of cytokines participate in repair after skin injury. For example, neutrophils secrete inflammatory cytokines including tumor necrosis factor-α TNF-α , IL-1β and IL-6 Burzynski et al.
TGF-β secreted by platelets can also promote the recruitment of inflammatory cells during inflammation. Moreover, once the wound area is disinfected. TGF-β can deactivate superoxide production from macrophages which helps to protect the surrounding healthy tissue and prepares the wound for granulation tissue formation Barrientos et al.
PDGF, and bFGF secreted by platelets and macrophages, respectively, can promote the migration of fibroblasts from the nearby dermis to the wound surface and accelerate the formation of granulation tissue to fill the gap in the wound Su et al. In addition, EGF can attract keratinocytes to migrate to the wound site and stimulate the wound to re-epithelialize Wang et al.
These cytokines all play different roles in the skin repair process. How to incorporate cytokines into the hydrogel to accelerate skin repair has become a research hotspot. Xuan et al. The content of bFGF released in situ stimulated the proliferation and migration of keratinocytes, endothelial cells, and fibroblasts, promoting collagen formation and re-epithelialization at the wound site.
An ethylene glycol chitosan hydrogel containing EGF and bFGF was cured by visible light. EGF stimulated the migration of fibroblasts into the wounded area while bFGF accelerated epithelial regeneration, granulation tissue growth, and collagen formation Yoo et al.
Compared with pristine ethylene glycol chitosan, the growth factor-loaded hydrogels exhibited better wound healing efficacy. Different cells play various regulatory roles in the wound healing process. At the inflammation stage, the successively infiltrating neutrophils, macrophages, and lymphocytes clear the invading microorganisms and cell debris and release inflammatory mediators.
In the proliferation stage, fibroblasts and endothelial cells promote capillary growth, collagen formation, and granulation tissue development in the wounded area. Keratinocytes proliferate from the edge of the wounds and migrate to the wound areas to promote epidermal formation.
However, deficient proliferation and fibroblast, keratinocyte, and endothelial cell migration will delay wound healing. NO can promote the migration and proliferation of cells related to wound healing.
The drug-loaded hydrogel showed a significant increase in cell proliferation and faster recovery of the scratched wound area Zahid et al.
In vitro cell migration tests showed that the cell migration rate in response to scratches increased by four-fold compared to the control samples. The application of chitosan hydrogels has been limited by their relatively poor mechanical properties.
Therefore, improving the mechanical properties of chitosan hydrogels has become a research pursuit. Lignin-chitosan-polyvinyl alcohol composite hydrogel was shown to possess better mechanical strength than primitive chitosan due to the introduction of lignin into the molecule Zhang et al. Lignin is a 3D reticulated biomacromolecule containing active sulfonate and phenolic hydroxyl groups.
As a major component of the plant cell wall, lignins can increase the structural strength of plant cells and improve their resistance to an adverse environment.
Moreover, the sulfonate groups in lignin can form ionic bonds with the amino groups of chitosan, further increasing the mechanical strength of the composite hydrogels.
In addition, wound dressings with special functions have been developed for diabetic wound treatment. Long-term anoxia is one of the main factors impeding the healing of diabetic wounds Patil et al.
The pO 2 of healthy skin ranges between 10 and 40 mmHg, whereas the pO 2 of the non-wounded skin of diabetic patients is as low as 5 mmHg Mutluoglu et al.
A previous study reported that a pO 2 value of 25— mmHg was needed for collagen synthesis, angiogenesis, and epithelial regeneration Mutluoglu et al.
An oxygen-containing biomaterial, MACF chitosan hydrogel, was manufactured and verified to enhance oxygen transportation, thereby promoting collagen synthesis and collagenous fiber rearrangement in diabetic wounds, which are processes beneficial to wound repair Patil et al.
Skin wounds are an extremely common and result from a variety of traumas, such as microbial infections, ulcers, tissue dehydration, and secondary damage. Thus, the development of functional wound dressings capable of promoting wound healing is of great clinical importance.
As a kind of native polysaccharide, chitosan is commonly used as wound care material due to its antibacterial and hemostatic properties, as well as the ability to promote granulation tissue growth.
Compared to other forms, hydrogels possess the advantages of tissue adhesion and water-retaining properties, and the ability to provide physical barriers for wounds. In order to improve the effect of chitosan-based hydrogel in accelerating wound healing, researchers have taken many efforts in recent years.
For example: 1 Chemical modifications by introducing hydrophilic groups onto chitosan molecules have increased its solubility. Methods such as acylation, carboxylation, quaternization, etherification, and alkylation are often used to introduce aliphatic acyl groups, aromatic acyl groups, carboxyalkyl groups, and quaternary ammonium groups into the molecular structure of chitosan.
This type of hydrogel is widely used in drug-release control, tissue engineering scaffold materials, and medical excipients, and is currently an active research topic.
This type of hydrogel can self-heal to reform a complete shape after a rupture to provide continuous and effective treatment of wounds.
A number of studies verified that the hydrogels containing these inorganic nanoparticles displayed excellent antibacterial effects against Gram-negative E. coli and Gram-positive S. aureus bacteria. The 3D network of chitosan hydrogel can accomplish storage promote the proliferation and migration of keratinocytes, endothelial cells, and fibroe and fixation of the drug.
Whilst, the hydrophilic network of chitosan hydrogels provide an adaptive environment for cells, improving their survival rate during transplantation. Nevertheless, there are still some problems for chitosan-based hydrogels to be solved.
For example, the poor solubility of chitosan and poor mechanical properties of hydrogels limit their applications in medical devices. Components like drugs, nanoparticles and other substances that were incorporated into the hydrogels make them possess the antibacterial functions. However, certain cytotoxic issues are produced at the same time.
How to improve the solubility for chitosan materials to be easily performed or shaped, to minimize the toxicity and to enhance the all advantages while maintaining their intrinsic natures are to be necessary for further investigation.
We look forward to the development of multifunctional chitosan hydrogels with promising prospects in wound treatment. PF and YL wrote the manuscript.
CK, HQ, and WW corrected and filed up the references. YZ designed and corrected the whole manuscript. RH, LX, and SW proposed the information. All authors contributed to the article and approved the submission.
This work was also sponsored by K. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. coli, Escherichia coli ; EGF, epidermal growth factor; FTIR, Fourier transform infrared spectroscopy; GCH, glycol chitosanhydrogel; GNB-OM, Gram-negative bacterial outer membrane; GP, glycoprotein; HA, hyaluronic acid; HBC-RGD, hydroxybutyl chitosan-Arg-Gly-Asp; HPMC, hydroxypropyl methylcellulose; HUCMSC-exos, umbilical cord-derived mesenchymal stem cell-derived exosomes; HUVECs, human umbilical vein endothelial cells; IL-1, interleukin-1; IL-6, interleukin-6; IL-8, iInterleukin-8; MACF, methacrylamide fluoride; MBA, methylene bisacrylamide; MDR, multidrug-resistant; MMRSA, Mupirocin-methicillin-resistant Staphylococcus aureus ; NO, nitric oxide; NPs, nanoparticles; OCNPs, oleoyl-chitosan nanoparticles; OKGM, oxidized konjac glucomannan; P.
aureus, Staphylococcus aureus ; S. cerevisiae, Saccharomyces cerevisiae ; S. pyogenes, Streptococcus pyogenes ; S. typhimurium, Salmonella typhimurium ; SA, sodium alginate; SEM, scanning electron microscopy; SeNPs, selenium nanoparticles; SNAP, S -nitroso- N -acetyl- DL -penicillamine; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; TOCNF, tempo-oxidized cellulose nanofiber; TXA2, thromboxane A2; VEGF, vascular endothelial growth factor; VWF, Von Willebrand factor; ZnO, zinc oxide.
Abd El-Hack, M. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Bagher, Z. Drug Deliv. CrossRef Full Text Google Scholar. Barrientos, S. Perspective article: growth factors and cytokines in wound healing. Wound Repair Regenerat. Bernkop-Schnürch, A. Strategies to overcome the polycation dilemma in drug delivery.
Blacklow, S. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Burzynski, L. The coagulation and immune systems are directly linked through the activation of interleukin-1α by thrombin.
Immunity 50, — Cheng, N. Acta Biomater. Clifton, L. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 31, — Fang, F. Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling.
Farhadihosseinabadi, B. Crosstalk between chitosan and cell signaling pathways. Life Sci. Fukasawa, M. The hemostatic effect of deacetylated chitin membrane on peritoneal injury in rabbit model. Today 22, — Galván Márquez, I. Disruption of protein synthesis as antifungal mode of action by chitosan.
Food Microbiol. Golmohammadi, R. Synergistic antibacterial activity and wound healing properties of selenium-chitosan-mupirocin nanohybrid system: an in vivo study on rat diabetic staphylococcus aureus wound infection model.
Guilak, F. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. Guo, Z. Nanobiohybrids: materials approaches for bioaugmentation. Haddadi, G. Evaluation of the effect of hesperidin on vascular endothelial growth factor gene expression in rat skin animal models following cobalt gamma irradiation.
Cancer Res. He, J. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. He, Q.
Positive charge of chitosan retards blood coagulation on chitosan films. Helander, I. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Howling, G. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro.
Biomaterials 22, — Huang, W. On-Demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl. Interfaces 10, — Jackson, S.
PI 3-kinase pβ: a new target for antithrombotic therapy. Jayaramudu, T. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications.
Jiang, Y. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Kassem, A. Antibacterial activity of chitosan nano-composites and carbon nanotubes: a review. Total Environ. Khan, M.
A review on recent advances in chitosan based composite for hemostatic dressings. Google Scholar. Khorasani, M. Kumar, A. Lambers, H. Natural skin surface pH is on average below 5, which is beneficial for its resident flora.
Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Leonhardt, E. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Li, M.
Two-Pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Li, X. Supramolecular antibacterial materials for combatting antibiotic resistance.
Li, Z. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Lord, M. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins.
The wound had been present for approximately one month, with previous localised treatment involving wound dressing with povidone-iodine solution.
As part of the wound management methods, debridement of the wound area was performed to manage exudate levels and infection, and to maintain the moisture level of the wound. Debriding also prevents the spread of infected tissue, hence enabling the rate of recovery of the wound to increase.
As part of the previous treatment regimen, a povidone-iodine solution-soaked gauze had been applied to prevent infection on the wound area, and surgical debridement had been performed, due to persistent infections, along with fluid treatment and other supportive treatments to manage the patient's condition.
Previous treatment of hernia had included surgical repair through the anterior approach, using an incision at the umbilicus. At the time of admission, the wound area was 60×50mm with a depth of 40mm. Throughout the course of treatment, chitosan dressings were used and changed on alternate days, with a total of 30 dressings used 10×10cm.
Due to the previous lack of improvement in wound healing with use of standard povidone-iodine dressings, this advanced bioactive microfibre dressing was used to manage the complications of this hard-to-heal wound infection. It is a highly absorbent wound dressing which has haemostatic, antimicrobial and pain relief properties, as well as the property of managing its exudate.
During 60 days of treatment with the new-generation dressing Fig 1 , healing of the wound had progressed significantly, despite the complex conditions of the wound high level of exudate, infection, depth and pain.
The results achieved with the use of a chitosan dressing were reduction in pain and exudate levels, along with reduction of infection. The ease of application and removal of the dressing and comfort level of the patient with use of this dressing were found to be excellent. The patient reported that the dressing was comfortable to wear.
The wound closed completely after 60 days of treatment. The patient gave written consent for the case details and photographs to be published. Because the dressing was already on the market and approved for wound healing, there was no requirement to gain ethical approval. Incisional hernia is mostly caused by abdominal surgery.
However, surgical meshes can trigger various responses when implanted in the human body, including inflammation foreign body reaction , fibrosis, calcification, thrombosis and infection. Meshes made of non-absorbable polymers have been used most frequently in clinical practice. Recent studies have indicated that the weight of the mesh and its pore size also have a bearing on mesh-related reactions in the body.
The patient presented with infection on the surgical site within a week and the wound grew larger within a month. The povidone-iodine dressings did not resolve the problem in this patient's case, and so the authors switched to the advanced wound care dressing based on chitosan.
The dressing used is an advanced wound dressing which works on the principle of bioactive microfibre gelling technology.
It absorbs excess exudates and creates an ideal environment for wound healing. Upon contact with wound exudates, it transforms into a cohesive gel matrix that conforms to the wound bed, maintains optimal moisture balance, eliminates empty spaces and provides a soothing effect at the wound site.
The wound was infected and had been present for the previous month on the surgical site when the chitosan dressing application was initiated. Chitosan possesses broad-spectrum antibacterial activity and has been shown to be active not only in soluble form but also in insoluble forms, such as nanoparticles and in wound dressings.
This forms a three-dimensional polymer network that can absorb large amounts of water and is moist, flexible and soft, conforming to the contours of the wound.
During contact with the wound, the gelling chitosan dissolves slowly in the microenvironment, releasing chitosan molecules which act as antibacterial agents, preventing bacterial growth and reducing infections in the wound.
The antibacterial properties of chitosan in solution are well described in the literature. Chitosan helps in the wound healing process by stimulating inflammatory cells, macrophages and fibroblasts during the inflammatory phase of the wound healing process. In our case, the patient had developed an infection at the suture line, causing multiple challenges to manage the rate of healing of the wound; however, the chitosan dressing showed remarkable results for management of exudate, infection and pain.
Chitosan has also proven to be an effective analgesic and literature reports suggest that chitosan absorbs the hydrogen ions at the inflammatory site and reduces the pain experienced by the patient. The results with the use of chitosan dressing showed a faster rate of wound healing as compared to previously used povidone-iodine dressings.
This case represents the advantages of chitosan-based advanced wound care dressing in surgical wounds and suggests that the use of chitosan-based dressings can be helpful in preventing complications and promoting healing in post-surgical wounds.
The chitosan dressing was started one month after the surgery. At this time the wound was already infected and inflamed. The exudates and the presence of necrotic tissue are other factors to be considered in the use of these dressings. After chitosan dressing use the wound closed within 60 days.
In our opinion, chitosan dressings are best suited for heavily exudating wounds irrespective of their stage. Leg ulcers. Surgical site infection. Wound dressings. Use of chitosan wound dressing for the treatment of surgical site infection: a case report.
a Orthopaedic Medical Center, The Second Protein intake for better digestion of Chitoasn University, ChangchunP. China E-mail: heliu ciac. cncathwang hotmail. comevanlee comqinyanguo hotmail. comwangzhjlu outlook. comqq.Video
Rainhome Medical Chitosan Wound Dressing Increasing mental focus wounds not dressimg cause physical Protein intake for better digestion for patients but dound are drdssing economic burden for society. It is necessary to seek out an efficient approach Fat intake and omega- promote skin repair. Hydrogels are Chitosn effective wound dressings. They possess many unique properties like biocompatibility, biodegradability, high water uptake and retention etc. Chitosan is a polymeric biomaterial obtained by the deacetylation of chitin. With the properties of easy acquisition, antibacterial and hemostatic activity, and the ability to promote skin regeneration, hydrogel-like functional wound dressings represented by chitosan and its derivatives have received extensive attentions for their effectiveness and mechanisms in promoting skin wound repair.
Diese Phrase ist einfach unvergleichlich:), mir gefällt)))