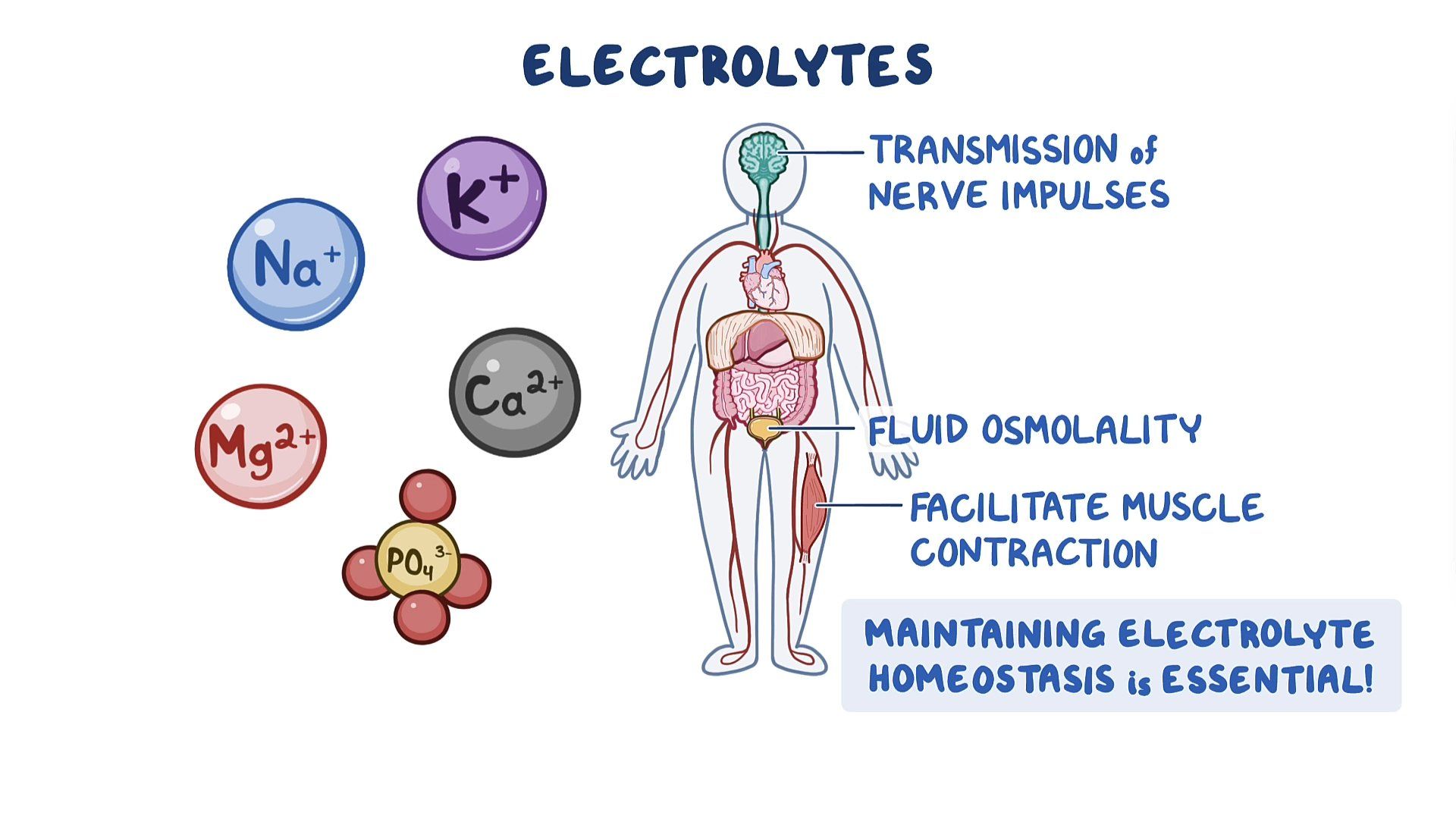
Electrolyte balance significance -
In hypotonic dehydration, intravascular water shifts to the extravascular space and exaggerates the intravascular volume depletion for a given amount of total body water loss. Neurological complications can occur in hypotonic and hypertonic states. The former can lead to seizures, while the latter can lead to osmotic cerebral edema upon rapid rehydration.
In more severe cases, the correction of a dehydrated state is accomplished by the replenishment of necessary water and electrolytes through oral rehydration therapy or fluid replacement by intravenous therapy.
As oral rehydration is less painful, less invasive, less expensive, and easier to provide, it is the treatment of choice for mild dehydration.
Solutions used for intravenous rehydration must be isotonic or hypotonic. Cell electrolytes : This diagram illustrates the mechanism for the transportation of water and electrolytes across the epithelial cells in the secretory glands.
Sodium is an important cation that is distributed primarily outside the cell. The total body sodium, however, is about 3, mmol as there is about 1, mmol stored in bones. Extra sodium is lost from the body by reducing the activity of the renin —angiotensin system that leads to increased sodium loss from the body.
Sodium is lost through the kidneys, sweat, and feces. In states of sodium depletion, the aldosterone levels increase. In states of sodium excess, aldosterone levels decrease. The major physiological controller of aldosterone secretion is the plasma angiotensin II level that increases aldosterone secretion.
Renin—angiotensin system : The regulation of sodium via the hormones renin, angiotensin, and aldosterone. In states of sodium depletion, the aldosterone levels increase, and in states of sodium excess, the aldosterone levels decrease. A low renal perfusion pressure stimulates the release of renin, which forms angiotensin I that is converted to angiotensin II.
Angiotensin II will correct the low perfusion pressure by causing the blood vessels to constrict, and increase sodium retention by its direct effect on the proximal renal tubule and by an effect operated through aldosterone. The perfusion pressure to the adrenal gland has little direct effect on aldosterone secretion and the low blood pressure operates to control aldosterone via the renin—angiotensin system.
Aldosterone also acts on the sweat ducts and colonic epithelium to conserve sodium. When aldosterone is activated to retain sodium the plasma sodium tends to rise. Potassium is predominantly an intracellular ion.
Most of the total body potassium of about 4, mmol is inside the cells, and the next largest proportion — mmol is in the bones. Extracellular potassium is about 4. In an unprocessed diet potassium is much more plentiful than sodium.
It is present as an organic salt, while sodium is added as NaCl. The body buffers the extra potassium by equilibrating it within the cells. The acid—base status controls the distribution between plasma and cells. A high pH i. A high plasma potassium level increases aldosterone secretion and this increases the potassium loss from the body to restore balance.
Therefore, a person with an acidosis pH 7. This occurs in diabetic acidosis. Calcium is a very important electrolyte. Ninety-nine percent or more is deposited in the bones and the remainder plays a vital role in nerve conduction, muscle contraction, hormone release, and cell signaling.
The solubility product of Ca and P is close to saturation in plasma. Even if it was all soluble it is not all absorbed as it combines with phosphates in the intestinal secretions.
Absorption is controlled by vitamin D while excretion is controlled by parathyroid hormones. However, the distribution from bone to plasma is controlled by both the parathyroid hormones and vitamin D.
There is also a constant loss of calcium via the kidneys even if there is none in the diet. This excretion of calcium by the kidneys and its distribution between bone and the rest of the body is primarily controlled by the parathyroid hormone. It is the ionized calcium concentration that is monitored by the parathyroid gland —if it is low, parathyroid hormone secretion is increased.
Any excess is excreted by the kidney and this excretion is increased by the parathyroid hormone. Electrolyte levels can change in relation to water levels in the body, as well as other factors. Important electrolytes, including sodium and potassium, are lost in sweat during exercise. A rapid loss of fluids, such as after a bout of diarrhea or vomiting, can also affect the concentration of electrolytes.
In these types of situations, the balance of electrolytes in the body needs to be restored. The kidneys and several hormones regulate the concentration of each electrolyte. If the level of one is too high, the kidneys filter it from the body, and different hormones act to restore a balance.
An imbalance causes a health issue when the concentration of a certain electrolyte becomes higher than the body can regulate. Low levels of electrolytes can also affect overall health. The most common imbalances involve sodium and potassium.
The symptoms depend on which electrolyte is out of balance and whether its level is too high or too low. A harmful concentration of magnesium, sodium, potassium, or calcium can produce one or more of the following symptoms:.
For example, a calcium excess can occur in people with breast cancer , lung cancer , or multiple myeloma. This type of excess is often caused by the destruction of bone tissue. As these symptoms can also result from cancer or cancer treatment, it may be difficult to identify high calcium levels as the cause.
An electrolyte panel is a test that screens for imbalances in the blood. It also measures the acid-base balance and kidney function. This test can help monitor the progress of treatment relating to a known imbalance. A doctor may include it as part of a routine physical exam, and people often undergo it during a hospital stay or when receiving care in an emergency room, as both acute and chronic illnesses can affect electrolyte levels.
A healthcare professional may also perform this test for someone taking medication known to affect electrolyte concentrations, such as diuretics or angiotensin converting enzyme inhibitors.
The levels of electrolytes in the blood are measured in millimoles per liter l. If the level of one type of electrolyte is too high or low, the doctor will test regularly until the levels are back to normal. If there is an acid-base imbalance, the doctor may carry out blood gas tests.
These measure the acidity, oxygen, and carbon dioxide levels in a sample of blood from an artery. They also determine the severity of the imbalance and how the person is responding to treatment. Treating an electrolyte imbalance involves either restoring levels that are too low or reducing concentrations that are too high.
If levels are too high, the treatment depends on the cause of the excess. If the body loses water without losing electrolytes, this can lead to an excess, and the treatment involves an infusion of water and glucose.
Healthcare professionals typically treat low levels by supplementing the needed electrolyte. The type of treatment will also depend on the severity of the imbalance. However, the symptoms of an imbalance can be severe, and a person may need to be hospitalized and monitored during the treatment.
Doctors mainly use this to treat an electrolyte shortage alongside dehydration, which tends to follow severe diarrhea.
The World Health Organization WHO has approved a solution for oral rehydration therapy that contains:. In more severe cases of an electrolyte shortage, healthcare professionals may administer the electrolyte orally or through an IV drip. An infusion of saltwater solution or compound sodium lactate, for example, can help treat a shortage of sodium.
Some causes of an electrolyte shortage, such as kidney disease, are not preventable. In general, having a well-managed diet can help reduce the risk of low electrolyte levels. Also, having a moderate amount of a sports drink during or after any kind of exertion or exercise can help limit the effects of losing electrolytes through sweat.
The consumption side is regulated by behavioral mechanisms, including thirst and salt cravings. While almost a liter of water per day is lost through the skin, lungs, and feces, the kidneys are the major site of regulated excretion of water. One way the the kidneys can directly control the volume of bodily fluids is by the amount of water excreted in the urine.
Either the kidneys can conserve water by producing urine that is concentrated relative to plasma, or they can rid the body of excess water by producing urine that is dilute relative to plasma.
Direct control of water excretion in the kidneys is exercised by vasopressin, or anti-diuretic hormone ADH , a peptide hormone secreted by the hypothalamus.
ADH causes the insertion of water channels into the membranes of cells lining the collecting ducts, allowing water reabsorption to occur. Without ADH, little water is reabsorbed in the collecting ducts and dilute urine is excreted.
ADH secretion is influenced by several factors note that anything that stimulates ADH secretion also stimulates thirst :. By special receptors in the hypothalamus that are sensitive to increasing plasma osmolarity when the plasma gets too concentrated.
These stimulate ADH secretion. By stretch receptors in the atria of the heart, which are activated by a larger than normal volume of blood returning to the heart from the veins.
These inhibit ADH secretion, because the body wants to rid itself of the excess fluid volume. By stretch receptors in the aorta and carotid arteries, which are stimulated when blood pressure falls.
These stimulate ADH secretion, because the body wants to maintain enough volume to generate the blood pressure necessary to deliver blood to the tissues.
Carb counting for athletes play a vital role in Electrolyte balance significance homeostasis within the body. They Electrolyte balance significance regulate myocardial and neurological function, Elecgrolyte balance, oxygen delivery, Electrolyte balance significance balance, and other biological processes. Electrolytes significznce important because Electropyte are what cells especially those of the nerve, heart, and muscle use to maintain voltages across their cell membranes and to carry electrical impulses nerve impulses, muscle contractions across themselves and to other cells. Electrolyte imbalances can develop from excessive or diminished ingestion and from the excessive or diminished elimination of an electrolyte. The most common cause of electrolyte disturbances is renal failure.
Sie hat der ausgezeichnete Gedanke besucht
Ich beglückwünsche, die ausgezeichnete Idee und ist termingemäß