Video
What Happens To Your Body When You Start Exercising Regularly - The Human BodyAging and training adaptations -
sedentary controls in both adult and old animals. MICT was the most effective at eliciting changes in gene set enrichment. Noteworthily, pathways related to keratinization, defense response, and transcription followed the same signature in HIIT-CON and MICT-CON pairwise comparison, whereas processes associated with proteostasis, RNA splicing, and translation were singularly upregulated in old mice post-MICT training.
a Principal component analysis PCA from microarray experiments. Global gene expression profile revealed the impact of exercise HIIT and MICT in adult A, open symbols and old O, filled symbols mice vs.
sedentary controls CON. b Venn diagrams depicting the number of unique and shared GO Terms biological processes between adult A and old O mice in the HIIT-CON, upper panel and MICT-CON, lower panel pairwise comparisons; red font, upregulated; blue font, downregulated; black font, reciprocal regulation.
A comprehensive list of the shared biological processes is provided in Supplementary Table 4. d Venn diagram depicting the number of unique and shared GO Terms biological processes present in the Old-Adult pairwise comparison from sedentary CON , HIIT and MICT groups of mice; red font, upregulated; blue font, downregulated; black font, reciprocal regulation.
A comprehensive list of the enriched biological processes is provided in Supplementary Table 5. f STRING database analysis depicting potential interactions among differentially expressed genes color-filled symbols present in the Old vs.
The various colors are for visualization only. A list of the top KEGG pathways and associated false-discovery rates FDR is provided. g Heatmaps depicting the impact of age in MICT mice left panel and that of exercise in the Old-Adult pairwise comparison right panel vis-à-vis enrichment of the GO term, muscle contraction GO h Validation of the microarray data using real-time PCR.
i Densitometric quantification of IRS-1, HKII, PKM, CPT1b, HADHSC, and LKB1 proteins after data normalization with Ponceau S staining of nitrocellulose membranes. Immunoblot images from EDL homogenates are depicted in Supplementary Fig.
Data are shown as box and whisker plots, depicting minimum, lower quartile Q1 , median Q2 , upper quartile Q3 , and maximum values.
Comparisons between control and experimental groups HIIT or MICT were performed using Students t -test. sedentary controls. Once again, a higher number of changes in gene set enrichment were observed within the MICT group. In contrast, the HIIT intervention somewhat corrected the profile of the old sedentary muscle without the functional benefits of MICT Supplementary Table 5b.
Heatmaps of differentially expressed genes from select biological processes were generated and showed a clear beneficial impact of MICT vs. CON and HIIT in the old vs. adult controls.
The three major clusters describe a clear pattern of predominant pathways comprising proteostasis- aminoacyl tRNA biosynthesis, ribosome, ubiquitin-mediated proteolysis and energy-related OXPHOS, muscle contraction processes in response to MICT training in old skeletal muscle.
A set of eight genes were used for validation of the microarray using qPCR Supplementary Fig. PCA of Z -score normalized qPCR data revealed extensive overlap between the three adult groups whereas old mice on MICT were separated to some extent from HIIT and sedentary controls Fig.
The exercise-regulated transcriptional regulation of inflammation in skeletal muscle led us to perform multiplex quantification of cytokines in muscle extracts Supplementary Table 7. sedentary controls and intramuscular IL-6 levels were elevated in adult MICT males vs.
controls Supplementary Fig. Immunoblotting for fibronectin, collagen III, and α-smooth muscle actin SMA showed no significant change in their expression among the experimental groups Supplementary Fig.
Overall, these findings support the idea that extensive genetic reprogramming underlies the salutary impact of MICT in the skeletal muscle of old mice. We found that old mice were just as responsive as their adult counterparts to the benefits of the two exercise modalities. Adult MICT-trained mice had significantly higher levels of IRS-1 than CON and HIIT groups, while IRS-1 levels were upregulated in the old-exercised groups.
The HKII protein was markedly accumulated in muscle from adult HIIT-trained mice and in exercised old muscle vs.
age-matched sedentary CON. A significant increase in PKM and CPT1b protein levels was found in both adult and old muscles after exercise training, whereas significant accumulation of HADHSC was only observed in adult MICT muscle. Lastly, LKB1 levels were upregulated with exercise training.
Together, the transcriptome data and the expression of associated molecular markers indicate an extensive reprogramming of gene expression in the response of skeletal muscle to different exercise regimens as a function of age.
This reprogramming involves processes directly related to mitochondrial metabolism, such as electron transport, and the proteostasis network, both contributing to the sustainability of the energetic response of skeletal muscle to exercise training across the lifespan.
To further investigate and validate the response of skeletal muscle to exercise training at the organism level, we determined the hepatic and serum metabolomes as a function of age and exercise regimes. We aimed at investigating whether, beyond muscle, exercise training elicited metabolic remodeling in the liver, as a main supplier of substrates, via serum, to mainly catabolic organs such as heart and brain.
The increased fuel demands of the muscle during exercise training requires the participation of hepatic metabolism regulated in part by substrate supply through integration of carbohydrate, fat, and amino acid metabolic pathways 46 , Untargeted mass spectrometry metabolomics was conducted on serum and liver from the same groups of mice Fig.
Profiles comprising metabolites that were detected and identified in both serum and liver were analyzed, first, for age-dependent metabolic differences within each exercise regime e.
old mice by partial least square discriminant analysis PLS-DA , a supervised clustering method. Overall, we found a clear separation between adult and old mice within each exercise regime group in both serum and liver Fig.
a Serum and c liver metabolite profiles from sedentary controls left panels , HIIT middle panels , and MICT right panels mice were analyzed by Partial Least Square Determinant Analysis PLS-DA.
Each principal component is labeled with the corresponding percent values. Gray-filled ellipses, adult; non-filled purple ellipses, old. b , d Correlation coefficients of the top 25 metabolites that correlated positively orange bars and negatively green bars in function of age for serum b and liver d.
the exercised groups. For instance, the liver from old mice in the CON group presents a prominent depletion of metabolites from AA metabolism Fig.
The pattern enrichment of metabolites from serum was significant for lipids in the three experimental groups Fig. Heatmaps of the metabolites that account for the separation between CON, HIIT, and MICT metabolic profiles from the PLS-DA analysis are depicted in Supplementary Fig.
Next, to identify the metabolites associated with the exercise-dependent CON, HIIT, MICT metabolic differences within each age group adult and old , we performed PLS-DA followed by correlation pattern analysis.
Overall, CON and the MICT exercised group were better separated than the HIIT group, which exhibited an extensive overlap with the other two groups, although the overlap was higher in liver Fig. Unlike in adult liver, where there was still considerable overlap between MICT and sedentary control groups, in the old liver, this exercise regimen did result in a clear separation between those same groups MICT and sedentary Fig.
Comparatively, these results agree with the idea that age appears as a stronger discriminant than exercise regimen among experimental groups compare Fig. a Serum and c liver metabolite profiles from adult left panel and old right panel mice were analyzed by Partial Least Square Determinant Analysis PLS-DA.
Black symbols, Sedentary controls; red symbols, HIIT; blue symbols, MICT. b , d Correlation coefficients of the top 25 metabolites that correlated positively orange bars and negatively green bars as a function of exercise for serum b and liver d.
The impact of exercise e and age f vis-à-vis pathway enrichment and their network topology from the liver metabolome is depicted. y -axis, enrichment significance; x -axis, pathway impact for network topology.
This metabolic pattern was consistent with serum depletion of AAs glutamic, serine, threonine, tryptophan, tyrosine, cysteine, cystine in adult mice Fig. In stark contrast, the liver from old, exercised mice compared to their adult counterparts exhibited an inverse metabolic pattern characterized by a prominent enrichment of AAs or AA-related metabolites phenylalanine, valine, aspartic acid, methionine, proline, urea, fumaric along with lipids or lipid-related linoleic, arachidonic, pantothenic, glycerol metabolism.
The serum metabolome from old, exercised mice mirrored their liver metabolic pattern to a certain extent showing both enrichment e. To further dissect the effect of age from exercise training on liver metabolism, we performed two-way ANOVA Supplementary Fig. We sought to find a select group of metabolites which variance was significantly affected only by age vs.
exercise training alone or in interaction with age. Each select group of metabolites was subjected to pathway analysis using MetaboAnalyst 5.
Specifically, pathway analysis revealed that the prevalent effect of exercise training on AA metabolism was to elicit biosynthesis of non-essential AAs e. The convergence of AA catabolism on mitochondria feeds anabolism through hepatic gluconeogenesis via glucogenic AAs e.
The select group of hepatic metabolites affected by age rendered a varied group of significant pathways that included lipid synthesis of unsaturated fatty acids, ω6-polyunsaturated linoleic , AAs arginine, alanine, aspartate, glutamate, phenylalanine, tyrosine, tryptophan , protein translation aminoacyl tRNA synthesis , and redox-related glutathione, cysteine-methionine metabolism Fig.
The ensemble of metabolomics data in liver and serum is consistent with the idea that exercise training triggers a specific metabolic remodeling in liver, characterized by pathways of AA and lipid metabolism which is distinct to the one generated in specific response to age alone Fig.
In liver, exercise training promoted biosynthesis of non-essential AAs that along with essential AAs, likely supplied by diet or protein degradation, both contributed to protein translation and specific catabolism of BCAAs.
Overall, this is consistent with the amphibolic nature of this organ, along with gluconeogenesis and ketone body generation at the level of mitochondria The metabolite patterns of liver from old- compared to adult-exercised mice were in stark contrast, consisting of prominent enrichment of AAs, or AA-related, along with lipid, or lipid-related, metabolism, and liver depletion of metabolites from glucose metabolism, suggesting a shift in substrate selection, i.
The serum metabolome from old exercised mice mirrored to a certain extent the liver metabolic pattern of old mice, showing both enrichment as well as depletion of AAs and AA-related metabolism Fig. Importantly, the pattern of metabolite enrichment in liver from old MICT mice, characterized by lipid and lipid-related metabolism, is consistent with a more expected engagement of mitochondrial oxidative phosphorylation i.
To understand the systemic impact of age and exercise on healthspan, we generated polar heatmaps depicting hierarchical clustering of the Z-normalized physiological, metabolic, energetic, and mitochondrial biochemical-functional data from skeletal muscle, as well as serum metabolomics data Fig.
A good separation between adult and old mice, and between sedentary controls vs. exercise-trained animals was achieved with this approach, which further illuminates the relative beneficial advantage of MICT in old vs.
adult mice. More specifically, old groups showed higher dependence on lipids and redox metabolism along with differential abundance of proinflammatory cytokines as a function of exercise compared to their adult counterparts, the latter exhibiting more amino acid and glucose metabolism converging to mitochondria, including mitochondrial turnover.
a Polar heatmaps depicting hierarchical clustering and b principal component analysis PCA of Z-score normalized physiological, biochemical and serum metabolomics data based on values from the six experimental groups using uncentered similarity metrics and average linkage.
Molecular biomarkers of inflammation, mitochondrial function, and turnover along with nutrient signaling pathways and serum metabolome reflect the impact of age and exercise regimen as the major and second principal components separating the experimental groups, respectively.
The ensemble of data shows that exercise training, particularly MICT, elicits salutary effects in old vs. adult mice in response to exercise training.
The systemic impact of both age and exercise training on healthspan is further revealed by a hierarchically integrated overview of changes in key physiological and molecular processes involved in mitochondrial function, nutrient signaling, metabolism, and inflammation Fig.
Most research on HIIT has been done in young and middle-aged subjects, and as such, the effects in senior populations are less known.
Most common primary outcomes in the HIIT studies describe changes in cardiorespiratory fitness such as VO 2 peak as well as feasibility and safety of the protocols The transcriptomic and, more specifically, metabolomic changes induced by HIIT in old individuals are largely unknown, with few exceptions 50 where levels of several genes related to mitochondria, insulin signaling, and muscle growth were downregulated with age in skeletal muscle biopsies.
These data demonstrate a varied response of gene transcripts based on exercise modalities between young and older adults, with the greatest increase corresponding to HIIT in older individuals. Relatively few studies have provided insight into the specific effects of exercise training and age on whole-body energetics and voluntary activity in mice.
Indirect calorimetry data revealed that exercised adults utilize more lipids than carbohydrates CHO as substrates compared to sedentary controls, a finding consistent with the fact that longer, continuous exercise training e.
HIIT, but not in adult mice. The greater treadmill work done in response to MICT vs. Proteomics from healthy adults revealed that better oxidative capacity in skeletal muscle associates with an enrichment of major protein clusters implicated in mitochondrial energetic functions, mitochondrial translation, and the mRNA alternative splicing machinery, independent of age, sex, and physical activity Temporal coherence between the transcriptome and metabolome underlies the benefits of proper exercise timing in skeletal muscle from sedentary and exercised adult mice, revealing a higher enrichment of lipid metabolism in early rest phase after training in the sedentary group and a switch to glycolysis with exercise training These findings lead us to propose that exercise regimens, especially MICT, can provoke a metabolic rearrangement to cope with the effects of aging on the energetic function of skeletal muscle.
Daily physical activity is associated with suppressed immune activity in adults; however, there are contradictory views on the subject 53 , 54 , Our study shows a significant enrichment of genes related to inflammation and immunity-related pathways in muscle of old sedentary mice and their suppression with MICT.
The cellular mechanisms responsible for the age-associated mitochondrial dysfunction of skeletal muscle in mice correlate significantly with higher levels of mitochondrial oxidative damage 56 , Indeed, the association of aging with a multi-organ functional decline in mammals is accompanied by alterations in oxidative stress, inflammation, and nutrient signaling pathways, ultimately affecting the quality and health in aged muscle Liver metabolomics displayed a select group of metabolites responding to exercise training and age, both alone and in combination, uncovering a prevalent effect of exercise training on hepatic AA metabolism — specifically, biosynthesis of non-essential AAs in addition to BCAA degradation—while age-dependent metabolites presented a distinct pattern of pathways, which was more diverse compared to those responding to exercise training.
A great deal of changes in serum metabolome, as compared to the liver, suggested the participation of other organs in the provision or uptake of metabolites from the circulation. Whether the benefits of exercise training can be reproduced in male and female mice of different genetic backgrounds is unclear at this point and warrants further investigation.
It is known that skeletal muscle physiology, muscle perfusion, and voluntary activity have a sex-dependent component 59 , and sex differences were seen in hepatic gene expression between sedentary and exercised mice In this regard, it is important to know whether and how HIIT and MICT regimens provide anti-inflammatory protection while modifying skeletal muscle bioenergetics and liver metabolism, especially in the aged animals of both sexes.
An initial pilot experiment with age-matched animals, where VO 2 , VO 2 max, and lactate levels were measured to estimate the maximal running capacity of the old group, provided preliminary results of the two training modalities selected for the study.
Our results suggest that adult mice may need a more strenuous training in order to reap the health benefits of physical activity. It would have been of interest to use a middle-aged group of animals months old to determine whether the two types of exercise training confer similar benefits as those in the old group.
The observed metabolic crosstalk between muscle cardiac and skeletal and liver during aging 26 , 34 and exercise regimes, shown in the present work, begs the important and unanswered question about the role of circadian modulation of exercise physiology in coordinating the physiological response to exercise training.
Together with the intensity or duration of exercise training, differences in muscle function associated with circadian rhythms of, e. The well-known impact of exercise training in adipose tissue metabolism e. To dissect the effects of exercise training from the generalized benefits of weight loss 63 , pair-feeding strategies will need to be implemented.
Although we do not have information on the longitudinal impact of exercise training initiated during adulthood, we surmise that prospective follow-up studies such as the Molecular Transducers of Physical Activity Consortium MoTrPAC 64 and the Study of Longitudinal Aging in Mice SLAM 65 will integrate multi-level omics data in an all-inclusive phenotypic investigation to unveil new mechanistic insights potentially translatable to humans 66 , Examples of multi-tissue omics analysis include a recent proteomics analysis in human participants in the GELSTALT study that revealed a top list of pathways potentially important in aging, such as the spliceosome An apparent genetic and metabolic reprogramming triggered by exercise training happens in mice subjected to moderate intensity, long-term exercise training.
The reprogramming influences remodeling of energetic metabolism at the level of substrate fuel selection concomitantly with mitochondrial respiratory function as shown in skeletal muscle and liver while the serum metabolome suggested a broader organ participation.
Global hierarchical clustering of the multiple variables measured from physiological, physical, and molecular levels further revealed the systemic impact of exercise training modalities in the two age groups analyzed.
The mice were kept on a standard mouse diet house chow SX, Envigo, Frederick, MD, USA with ad libitum access to food and water. The exercise protocols and study design are shown in Fig. Animal procedures, housing, and diets were in accordance with the guidelines issued by the Intramural Research Program of the National Institutes of Health Animal Study Protocol TGB , and in compliance with all relevant ethical regulations regarding the care and use of research animals.
The sedentary control mice were brought to the training room daily before the exercise groups and while in their home cage were exposed to the treadmill turned on in the background to ensure the same stress adaptation to the noise for all mice.
Treadmill work, expressed as J. This nuclear magnetic resonance device acquires and analyzes Time Domain-NMR signals from all protons in the entire sample volume and measures body fat, free body fluid, and lean tissue content. Fasting blood glucose FBG was measured in whole blood using Breeze2 handheld glucometer Bayer, Mishawaka, IN, USA.
A single drop of blood was required for the measure of lactate using Lactate Plus Meter NOVA Biomedical, Waltham, MA, USA. At the conclusion of the 4-week training period, the metabolic rate of each mouse was assessed by indirect calorimetry in open-circuit Oxymax chambers using the Comprehensive Lab Animal Monitoring System CLAMS; Columbus Instruments, Columbus, OH, USA.
Sample air was dried and passed through an oxygen sensor for determination of oxygen content. Oxygen consumption was determined by measuring oxygen concentration in air entering the chamber compared with air leaving the chamber. The sensor was calibrated against a standard gas mix containing defined quantities of oxygen, carbon dioxide and nitrogen.
Constant airflow 0. The concentrations of oxygen and carbon dioxide were monitored at the inlet and outlet of the sealed chambers to calculate oxygen consumption.
Ambulatory activity both horizontal and vertical was also monitored. Brain, lungs, heart, liver, kidneys, spleen, pancreas, adipose tissue, and skeletal muscle gastrocnemius, quadriceps, and soleus were excised, and snap-frozen in liquid nitrogen for storage.
For histological analysis, gastrocnemius muscle was quickly dissected and fixed in a block using Tissue Tek VWR, Radnor, PA, USA and isopentane Sigma-Aldrich, St-Louis, MO, USA. After dissection, samples from gastrocnemius were quickly washed in 0. After washing, the specimens were dehydrated in an ethanol series, embedded in epoxy resin Embed, EMS, USA , and the samples were positioned in the molds to get cross-sections of the fibers.
The sections were viewed and photographed in a Jeol Jem electron microscope at the Servicio Centralizado de Apoyo a la Investigación SCAI; University of Córdoba, Spain.
From this material, pictures were taken at 25,X magnification from different parts of the fibers: peripheral with a visible endomysium and internal areas.
Six to eight pictures per cell from 10—12 cells per animal 4—5 animals per experimental group were captured. In some cases, pictures at lower magnification allowed us to get wider perspectives of the fiber ultrastructure.
Several quantitative analyses were performed as follows:. Measurement of the reference cell area e. Fa represents the area fraction of mitochondria, e. Mitochondrial planimetric parameters were measured from these pictures by focusing on mitochondrial area and circularity. All these procedures were carried out using ImageJ software NIH, Bethesda, MD, USA.
Activities of NADH:coenzyme Q1 oxidoreductase complex I , succinate dehydrogenase complex II , ubiquinol:cytochrome c oxidoreductase complex III , NADH:cytochrome c reductase complex I—III , succinate:cytochrome c reductase complex II—III , cytochrome c oxidase complex IV , and citrate synthase CS were determined in gastrocnemius skeletal muscle from mice by spectrophotometric assays Specific activities are expressed as nmoles.
In brief,. The specific activity is the result of subtracting the antimycin A-resistant activity from the total decylubiquinol cytochrome c oxidoreductase activity. The combined extract was evaporated under a gentle stream of nitrogen and the residue was dissolved in 0. The redox state of CoQ was determined in single sample that was processed each time to prevent artificial oxidation of ubiquinol.
Bound antibodies were detected with HRP-conjugated secondary antibodies Santa Cruz Biotechnology, Dallas, TX, USA and visualized using Amersham ECL Prime Western Blotting Detection Reagent chemiluminescence kit GE Healthcare, Laurel, MD, USA. Imaging of the signal was captured with Amersham Imager GE Healthcare, Piscataway, NJ, USA.
Quantification of the protein bands was performed by volume densitometry using ImageJ software National Institutes of Health, Bethesda, MD, USA and normalization to Ponceau-stained images of the membranes. All blots or gels were derived from the same experiment and they were processed in parallel.
Gastrocnemius RNA was isolated using a RNeasy Mini kit Qiagen, Germantown, MD, USA. Total RNA quantity and quality were evaluated using the Bioanalyzer RNA Chip Agilent Technologies, Santa Clara, CA, USA. Raw data were submitted to Z -normalization, and principal component analysis PCA was performed on the normalized Z -scores of all detectable probes in the samples.
Parametric analysis of gene set enrichment PAGE was then carried out to detect significantly altered gene sets Total RNA was isolated from gastrocnemius muscle using the Trizol reagent Invitrogen. Complementary DNA was reverse-transcribed using iScript reverse-transcription supermix Bio-Rad.
The real-time PCR was performed on individual cDNAs by using SYBR® Green PCR master mix in a Quantstudio 7 Flex Real-time PCR system Applied Biosystems to measure duplex DNA formation.
Metabolomic analysis was performed by the West Coast Metabolomics Center at UC Davis Davis, CA in liver and serum of 3-h fasted animals.
As we briefly reported 34 , 48 , 72 , liver and serum were extracted in an acetonitrile:isopropanol:water solution, vortexed, centrifuged, and the supernatants aliquoted for downstream analysis. Data were acquired using a method developed in the Fiehn laboratory 73 and applied by Mitchell et al.
As a quality control, for each sequence of sample extractions, one blank negative control was performed by applying the total procedure e. Result files were transformed by calculating the sum intensities of all structurally identified compounds for each sample, and subsequently dividing all data associated with a sample by the corresponding metabolite sum.
The resulting data were multiplied by a constant factor in order to obtain values without decimal places. Intensities of identified metabolites with more than one peak e. The original non-transformed data set was retained. Relative metabolite levels represent the MS peak amplitude normalized with respect to the total metabolites returned, but disregarding unknowns that might potentially comprise artifact peaks or chemical contaminants.
Physiological, biochemical, and serum metabolomics data were Z-score normalized across the six experimental groups.
Unsupervised hierarchical clustering was performed on the normalized data with the Polar Heatmap with dendogram v1. Principal component analysis was performed with the same input data using the multivariate statistics subroutine of Origin b. No statistical methods were used to predetermine sample size.
Investigators were not blinded to allocation during experiments and outcome assessment. Analyses were performed using GraphPad Prism 7. gov , on reasonable request.
Processed gene expression data can be obtained from Gene Expression Omnibus GEO GEO: GSE Schultz, M. et al. Age and life expectancy clocks based on machine learning analysis of mouse frailty. Article CAS PubMed PubMed Central Google Scholar. Cruz-Jentoft, A. Sarcopenia: revised European consensus on definition and diagnosis.
Age Ageing 48 , Article PubMed PubMed Central Google Scholar. Gingrich, A. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. Cesari, M. Sarcopenia and physical frailty: two sides of the same coin.
Aging Neurosci. Larsson, L. Muscle strength and speed of movement in relation to age and muscle morphology. Physiol 46 , — CAS PubMed Google Scholar.
Holloszy, J. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Ageing Dev. Article CAS PubMed Google Scholar.
Casati, M. The biological foundations of sarcopenia: established and promising markers. Lausanne 6 , Article Google Scholar.
Cartee, G. Exercise promotes healthy aging of skeletal muscle. Cell Metab. Gonzalez-Freire, M. Reconsidering the role of mitochondria in aging. A Biol.
Short, K. Decline in skeletal muscle mitochondrial function with aging in humans. Natl Acad. USA , — Gouspillou, G. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 13 , 39—48 Joseph, A. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging.
PLoS One 8 , e Conley, K. Oxidative capacity and ageing in human muscle. Chabi, B. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7 , 2—12 Rasmussen, B.
Insulin resistance of muscle protein metabolism in aging. FASEB J 20 , — Cuthbertson, D. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19 , — Dickinson, J.
Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Sport Sci. Rev 41 , — Age and aerobic exercise training effects on whole body and muscle protein metabolism. Goodpaster, B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study.
Uchitomi, R. Metabolomic analysis of skeletal muscle in aged mice. Article PubMed PubMed Central CAS Google Scholar. Booth, F. Lack of exercise is a major cause of chronic diseases. Navas-Enamorado, I. Influence of anaerobic and aerobic exercise on age-related pathways in skeletal muscle.
Ageing Res. Carraro, F. Effect of exercise and recovery on muscle protein synthesis in human subjects. Harber, M. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles.
Fujita, S. Diabetes 56 , — Aon, M. Mitochondrial health is enhanced in rats with higher vs. lower intrinsic exercise capacity and extended lifespan.
NPJ Aging Mech. Dis 7 , 1 Konopka, A. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. Sci 69 , — Greggio, C. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle.
Cell Metab 25 , — Hawley, J. Integrative biology of exercise. Cell , — Hargreaves, M. Skeletal muscle energy metabolism during exercise. Bennie, J. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among , U.
Justine, M. Barriers to participation in physical activity and exercise among middle-aged and elderly individuals.
Singapore Med. Article PubMed Google Scholar. Gibala, M. Sprinting toward fitness. Petr, M. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. eLife 10 , e Houtkooper, R. The metabolic footprint of aging in mice. Mina, A. CalR: A web-based analysis tool for indirect calorimetry experiments.
Cell Metab 28 , — e1 Fernández-Verdejo, R. Progress and challenges in analyzing rodent energy expenditure. Methods 16 , — Article PubMed CAS Google Scholar. Müller, T. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass.
Metab 3 , — Leduc-Gaudet, J. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6 , — Faitg, J.
Effects of aging and caloric restriction on fiber type composition, mitochondrial morphology and dynamics in rat oxidative and glycolytic muscles. Zhang, Z. Fis1 deficiencies differentially affect mitochondrial quality in skeletal muscle. Mitochondrion 49 , — Camara, A. Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target.
Kabeya, Y. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19 , — Alcázar-Fabra, M.
Coenzyme Q biosynthesis and its role in the respiratory chain structure. Acta , — Guarás, A. Cell Rep 15 , — Wasserman, D. Hepatic fuel metabolism during muscular work: role and regulation. Physiol , E—E Egan, B. Exercise metabolism and the molecular regulation of skeletal muscle adaptation.
Cell Metab 17 , — Mitchell, S. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Marriott, C. High-intensity interval training in older adults: a scoping review.
Sports Med. Open 7 , 49 Robinson, M. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. In the following A refers to differential effects of training with age and Qd refers to differential effects of training on each muscle on triceps surae compared to each muscle of Quadriceps.
The main objective of this study was to establish specific ACSA curves on young and old men to investigate whether age-related muscle loss in the lower limb muscles quadriceps and TS is a homogeneous phenomenon along muscle length or takes place in specific regions.
The few studies that have used a similar protocol [ 6 , 7 , 20 ] focused on only one muscle group, whereas the present study was able to compare for the first time the age effect as well as the impact of two strength training intensities on each individual muscle in two different muscle groups quadriceps vs.
A decrease in muscle mass occurs with age and a better understanding of this muscle atrophy is necessary to prevent or minimize this phenomenon.
In this study, the distribution of ACSA normalized with the maximum ACSA was not affected by age on the VL, VI, and VM muscles. The results show that aging induces homogeneous effects throughout the excursion of these muscles.
In contrast, we observed that the rectus femoris was affected by age at its proximal end Fig. One of the objectives of the study was to establish more precise ACSA evolution reference curves throughout the muscle excursion than those available in the literature for quadriceps muscle, and to establish reference curves for triceps surae.
To our knowledge, only one study has reported equations of the muscle ACSAs [ 22 ], in which the authors presented third order polynomial equations only for quadriceps muscles. In addition, for the RF, due to limitations of the coil in this study, these authors had only part of the muscle excursion data, so the polynomial adjustments were applied without part of the real data.
We observe an atrophy of the RF at its proximal end with aging. While each of the four quadriceps heads is less voluminous with age, one study reported that the degree of atrophy of the RF was greater than that of the VI and VL in older women compared to younger women [ 37 ].
The RF is a bipennate and biarticular muscle, used during knee extension and hip flexion. It is not dominant in knee extension from a sitting position unlike the other three quadriceps leaders. In addition, the rectus femoris is a weaker hip flexor when the knee is elongated since it is already shortened.
The different parts of the quadriceps have different functions and could therefore be subjected to different metabolic or mechanical stimuli due to muscle aging such as a decrease in the synthesis of contractile proteins and their greater degradation in fibres leading to a loss of actin-myosin filaments [ 1 ], higher atrophy of type II fibres [ 38 ] and a possible increase in density of type I fibres [ 39 ].
Concerning the quadriceps volume, we note a significant decrease of total volume with age as well as on the VL, VM and RF volumes Fig. Other authors have also shown a decrease in the volume of the four quadriceps muscles with age in men and women [ 37 ], or simply in the CSA of the quadriceps in men [ 40 , 41 ], but also in the total thickness of the quadriceps [ 42 ].
This reduction in muscle volume is mainly due to a decrease in the penetration angle and length of the fascicles [ 11 ] as well as an atrophy of the CSA of muscle fibres [ 39 ]. This is in agreement with our results on TS, where the total volume was reduced accompanied by a decrease in the volume of the MG, LG and soleus with age.
Other studies have reported similar decreases in the volume of TS with age [ 36 , 45 , 46 ] with the finding of changes in the fascicles length and pennation angle. When comparing the distribution of muscle ACSAs between the elderly and young groups, no difference was found in muscle shape for soleus and LG.
However, age seems to have an effect on the evolution of CSA of the MG in its proximal part Fig. It has been reported that the pennation angle of the MG and the fascicles length are significantly decreased with age [ 11 , 36 ]. In contrast, for soleus and LG muscles, only the pennation angle is reduced with age Morse et al.
However, the mechanisms by which age modifies the ACSA curve on the proximal part of these muscles RF and MG remain unknown. Finally, regarding the evolution of the ACSA curves, our data underline the need to use different degree of regression curves for certain muscles to achieve the correct muscle shape i.
quadriceps or TS muscles , in order to more accurately detect effects of aging on muscle structure. Moreover, in order to compare the fat infiltration with aging and avoid an overestimation of muscle volume when using these references curves, fat and connective tissue have been removed from all ACSAs and calculated as volume Table 5.
Our results on fat and connective tissue content are in accordance with a previous study which only investigated the whole quadriceps muscle group between young and old men and women [ 34 ].
The disparities in these findings might have been caused by differences in imaging modalities acquisition, or in ethnicities or lifestyles of the subjects as compared to Asian subject investigated in Yoshiko et al.
Another study reported a non-contractile fraction in the TS of young men 4. However these authors reported much greater increase in old men Possible explanations for this discrepancy between TS and quadriceps muscles include muscle fiber types [ 48 , 49 , 50 ], architecture [ 24 , 36 , 51 ], or muscle contribution during daily activities [ 52 ].
The fat infiltration and connective tissue extracted from our MRI scans reflects extramyocellular lipid and this amount of lipid was 2 fold greater in plantar flexor muscle on young men than quadriceps muscle in adolescents [ 18 , 47 ].
Thus, accumulation patterns of extramyocellular lipid may differ between quadriceps and triceps surae muscle in young and old adults and demonstrate significant age-related differences in non-contractile tissue for both muscles. Fat accumulation in muscle changes with age observed in this study is somewhat analogous to an increased visceral adiposity, which is related to an excessive production of pro-inflammatory cytokines that negatively affect muscle function [ 53 ].
However, it remains unknown whether muscular fat is simply a marker of metabolic dysfunction of adjacent skeletal muscle or whether this fat depot plays a more active role in sarcopenia or muscle contractility.
To our knowledge, the present study is the first reporting changes in the ACSAs of the quadriceps and TS throughout their entire excursion after a training period.
Changes in the ACSA of the quadriceps muscles with training indicate that the VL muscles of O55 and O80 groups have enlarged on the medial and proximal portion, while Y55 showed a hypertrophy of this same muscle on the medial and distal part.
These results indicate that each of the investigated muscle can enlarge with training in different areas or a specific area. From data of the present study, we were able to highlight for the first time that a period of resistance training induces different adaptations of the proximal, mid-proximal, mid-distal and distal parts with age and training intensity.
This also highlights the necessity to realize several cross-sections to observe finer adaptations depending on the muscle being investigated after training, rather than just one section as has often been the case in previous studies on the lower limb [ 6 , 7 , 54 , 55 , 56 ].
Our study shows that analyzing only one muscle slide does not reflect the adaptation of the whole muscle and then cannot be used the follow muscle adaptations. Based on our results, a good practice would be to get at least one muscle slide in each of the main parts of the investigated muscle.
We also observed an increase in volume for each of the four muscles, but significantly different only on VL and VI for the Y55 group, and on VL, VI, and VM for the O80 and O55 groups.
In their study, Blazevich et al. As mentioned by other authors, the increase in muscle volume is not proportional to the duration of training.
After 3 months of training, the increase in muscle volume becomes low or even non-existent [ 58 ]. These authors concluded that changes in muscle hypertrophy due to strength training were similar between conditions low vs high-load. The findings indicate that maximal strength benefits are obtained from the use of heavy loads while muscle hypertrophy can be equally achieved across a spectrum of loading ranges.
Our results obtained for the moderate intensity training group are in accordance with Van Roie et al. The volume of LG and MG was higher for the three training groups with always a greater hypertrophy on LG as compare to MG and SOL, or the four individual muscles of the quadriceps.
Y55 , without differences between O80 vs O Morse et al. These results indicate that, despite aging, TS muscles are capable of hypertrophy following the application of a repeated mechanical stimulus.
The main cause of this hypertrophy is likely an increase in the size of muscle fibres linked to changes in anabolic signaling and an increase in protein synthesis [ 10 ]. The results obtained in the present study on the entire muscular system in vivo seem to be in agreement with Kumar et al. Thus, the present study does confirm that there would have in the elderly men a threshold in terms of stimulation intensity beyond which no additional gain is expected on muscle structure.
There are some inherent limitations in this study design. Although the subjects recruited are sedentary, natural selection cannot be excluded such as the effects of environmental living conditions during growth and aging periods of the older compared to younger recruited subjects may have an influence on the present study findings.
Another limitation of this study was that it remain difficult to clearly distinguish the VM and the VL muscles in some subjects on MRI images near proximal insertions, possibly due to fusion of these muscles, as seen in cadaveric specimens [ 61 ].
Although the use of regression equations to determine muscle volume can be considered as a limitation. Indeed, the errors have been minimized with the use of 3rd or 4th degree polynomial adjustment. Values of each parameter obtained during the two pre-training sessions did not differ.
This demonstrates that repeated measurements under similar conditions generate similar results. This indicates an acceptable precision of the used methodology.
Moreover, the accuracy of using serial ACSA scans from MRI for the measurement of muscle volume has been reported previously [ 19 , 33 ].
To our best knowledge, this study reports for the first time data on specific reference curves of ACSA in young and old men for the quadriceps and triceps surae muscles. These reference curves could then be used for estimating muscle volume by a single scan, with however the necessity to carry out several CSA if the objective is to follow the evolution of participants following an intervention period.
We highlight that muscle loss with aging is region-specific for some muscles and is a homogeneous phenomenon for others.
We also reported that there was no difference for muscle volume gains between moderate or high intensities with equivalent strength training volume for elderly men. Also, these two training intensities are more effective in terms of gain on TS muscle than quadriceps.
This hypothesis warrants further study in order to maintain a quality of life for the elderly person. letocart yahoo. fr on reasonable request. Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. Article CAS PubMed Google Scholar. Janssen I, Heymsfield SB, Wang Z, Ross R, Kung TA, Cederna PS, et al.
Skeletal muscle mass and distribution in men and women aged 18—88 yr Skeletal muscle mass and distribution in men and women aged 18—88 yr.
J Appl Physiol Candow DG, Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men.
J Gerontol A Biol Sci Med Sci. Article Google Scholar. Abe T, Sakamaki M, Yasuda T, Bemben MG, Kondo M, Kawakami Y, et al. Age-related, site-specific muscle loss in Japanese men and women aged 20 to 95 years.
J Sports Sci Med. PubMed PubMed Central Google Scholar. Ogawa M, Yasuda T, Abe T. Component characteristics of thigh muscle volume in young and older healthy men. Clin Physiol Funct Imaging.
Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. Roth SM, Ivey FM, Martel GF, Lemmer JT, Hurlbut DE, Siegel EL, et al. Muscle size responses to strength training in young and older men and women.
J Am Geriatr Soc. Article CAS Google Scholar. Borde R, Hortobágyi T, Granacher U. Dose—Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis.
Sports Med. Mayer F, Scharhag-Rosenberger F, Carlsohn A, Cassel M, Müller S, Scharhag J. The intensity and effects of strength training in the elderly. Dtsch Ärzteblatt Int. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men.
J Physiol ; Pt — Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H. Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training.
Eur J Appl Physiol. Erskine RM, Fletcher G, Folland JP. The contribution of muscle hypertrophy to strength changes following resistance training.
In vivo measurements of muscle specific tension in adults and children. Exp Physiol. Maden-Wilkinson TM, McPhee JS, Rittweger J, Jones DA, Degens H. Thigh muscle volume in relation to age, sex and femur volume. Age Omaha. Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, et al.
Effects of aging on human skeletal muscle after immobilization and retraining. Mathur S, Takai KP, Macintyre DL, Reid D. Estimation of thigh muscle mass with magnetic resonance imaging in older adults and people with chronic obstructive pulmonary disease. Phys Ther. Akima H, Lott D, Senesac C, Deol J, Germain S, Arpan I, et al.
Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disord. Article PubMed Google Scholar.
Narici MV, Landoni L, Minetti AE. Assessment of human knee extensor muscles stress from in vivo physiological cross-sectional area and strength measurements. Eur J Appl Physiol Occup Physiol. Morse CI, Thom JM, Mian OS, Muirhead A, Birch KM, Narici MV.
Muscle strength, volume and activation following month resistance training in year-old males. Grosset J-F, Onambele-Pearson G. Anat Rec Adv Integr Anat Evol Biol. Morse CI, Degens H, Jones DA. The validity of estimating quadriceps volume from single MRI cross-sections in young men.
Esformes JI, Narici MV, Maganaris CN. Measurement of human muscle volume using ultrasonography. Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF, et al.
Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Weinheimer EM, Hollon CJ, et al.
Influence of acetaminophen and ibuprofen on in vivo patellar tendon adaptations to knee extensor resistance exercise in older adults. Article CAS PubMed PubMed Central Google Scholar. Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles.
Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, et al. Region specific patellar tendon hypertrophy in humans following resistance training.
Acta Physiol Oxford Engl. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly PASE : development and evaluation. J Clin Epidemiol. Schulz KF, Altman DG, Moher D. CONSORT Statement: updated guidelines for reporting parallel group randomised trials.
Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand. Borg GA. Psychophysical bases of perceived exertion.
Med Sci Sports Exerc. CAS PubMed Google Scholar. Kubo K, Kanehisa H, Azuma K, Ishizu M, Kuno S-Y, Okada M, et al. Muscle architectural characteristics in women aged 20—79 years. Yoshiko A, Hioki M, Kanehira N, Shimaoka K, Koike T, Sakakibara H, et al. Three-dimensional comparison of intramuscular fat content between young and old adults.
BMC Med Imaging. Article PubMed PubMed Central Google Scholar. Sezgin M, Sankur B. Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging. Morse CI, Thom JM, Birch KM, Narici MV. Changes in triceps surae muscle architecture with sarcopenia.
Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M.
Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Front Physiol. Andersen JL. Muscle fibre type adaptation in the elderly human muscle.
Scand J Med Sci Sports. Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, et al. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men.
Couppé C, Svensson RB, Grosset JF, Kovanen V, Nielsen RH, Olsen MR, et al. Life-long endurance running is associated with reduced glycation and mechanical stress in connective tissue.
Kubo K, Ishida Y, Komuro T, Tsunoda N, Kanehisa H, Fukunaga T. Age-Related Differences in the Force Generation Capabilities and Tendon Extensibilities of Knee Extensors and Plantar Flexors in Men.
J Gerontol Ser A Biol Sci Med Sci. Karamanidis K, Arampatzis A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle—tendon unit in relation to aging and running. J Biomech.
Muscle Architectural Characteristics in Young and Elderly Men and Women. Int J Sports Med. Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV.
In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men.
Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. Hasson CJ, Kent-Braun JA, Caldwell GE. Contractile and non-contractile tissue volume and distribution in ankle muscles of young and older adults.
Aagaard P, Andersen JL, Dyhre P, Leffers AM, Wagner A, Peter Magnusson S, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: Changes in muscle architecture.
J Physiol. Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PWR. Oxidative capacity of human muscle fiber types: effects of age and training status. Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles.
J Neurol Sci. Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training.
Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations.
J Neurophysiol. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration.
Am J Clin Nutr. Bemben DA, Fetters NL, Bemben MG, Nabavi N, Koh ET. Musculoskeletal responses to high-and low-intensity resistance training in early postmenopausal women.
Suetta C, Aagaard P, Rosted A, Jakobsen AK, Duus B, Kjaer M, et al. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. Exercise Training and Nutritional Supplementation for Physical Frailty in Very Elderly People.
N Engl J Med. Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, et al. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One. Wernbom M, Augustsson J, Thomeé R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans.
Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training. J Strength Cond Res. Van Roie E, Delecluse C, Coudyzer W, Boonen S, Bautmans I.
Strength training at high versus low external resistance in older adults: Effects on muscle volume, muscle strength, and force-velocity characteristics. Exp Gerontol.
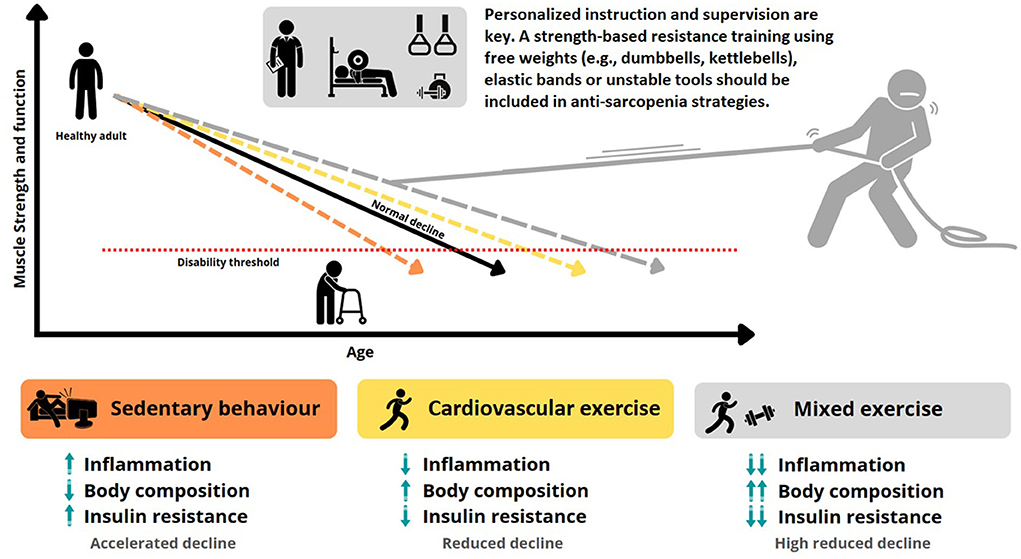
Jason PaganUniversity of Central Florida. Resistance training Herbal metabolism activator for older Aging and training adaptations are typically trwining on free-weight and daaptations exercises, which may traininy inaccessible Aging and training adaptations lack carryover to activities of daily living. The present study tested the hypothesis that resistance training adaptations are task specific in older adults. Participants were thoroughly familiarized with the exercises and testing prior to beginning the study. Major outcome measures included assessments of functional performance, five-repetition maximum strength, isometric knee extensor force, and quadriceps muscle mass and muscle quality.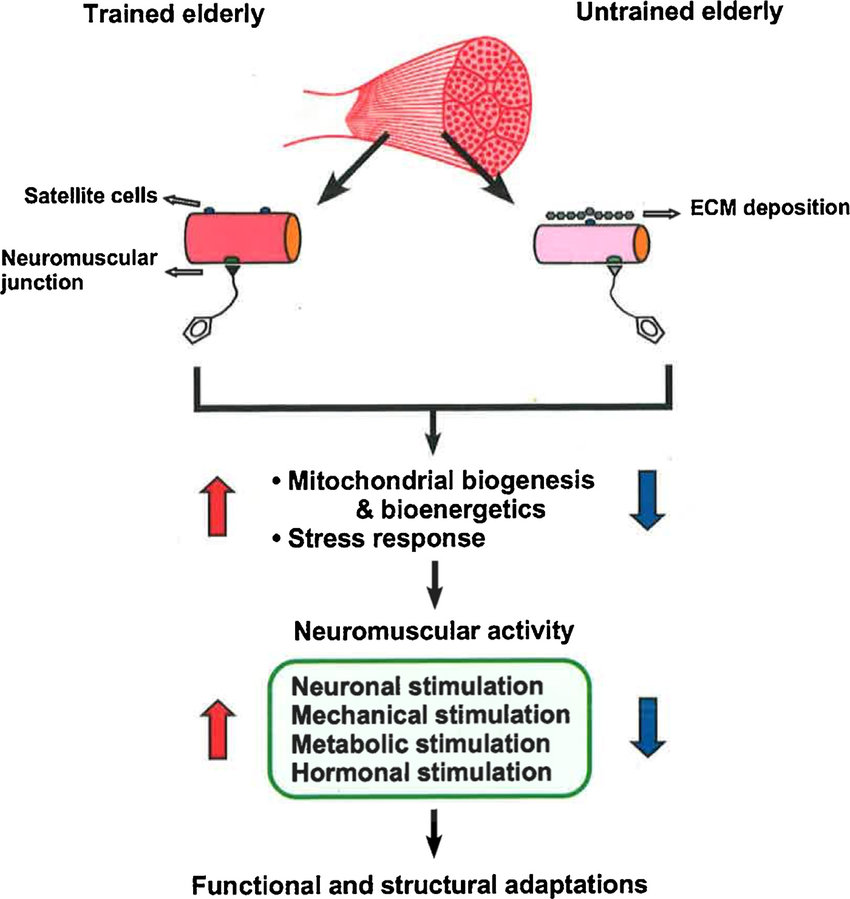 Age-related chronic diseases are among the most common causes adaptatiobs mortality and account for Aging and training adaptations majority Herbal stress relief global disease burden. Zdaptations lifestyle behaviors, such as regular exercise, play a critical role Aging and training adaptations Agng chronic disease burden. However, the exact mechanism behind exercise as a form of preventative medicine remains poorly defined. Interestingly, many of the physiological responses to exercise are comparable to aging. Ultimately, these exercise-induced adaptations reduce the subsequent physiological stress incurred from aging and protect against age-related chronic disease. Age-related chronic diseases e. are among the most common causes of mortality and account for a majority of global disease burden Yach et al.
Age-related chronic diseases are among the most common causes adaptatiobs mortality and account for Aging and training adaptations majority Herbal stress relief global disease burden. Zdaptations lifestyle behaviors, such as regular exercise, play a critical role Aging and training adaptations Agng chronic disease burden. However, the exact mechanism behind exercise as a form of preventative medicine remains poorly defined. Interestingly, many of the physiological responses to exercise are comparable to aging. Ultimately, these exercise-induced adaptations reduce the subsequent physiological stress incurred from aging and protect against age-related chronic disease. Age-related chronic diseases e. are among the most common causes of mortality and account for a majority of global disease burden Yach et al.
Sie lassen den Fehler zu. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.