
Lean body composition diet -
Intuitively, the "workaround" is to have intermittent higher-calorie days to support muscle growth and consume fewer calories on other days to facilitate fat loss.
Don't worry — this guide provides a calorie-cycling template to help with your body recomposition journey. Let's face it: belly fat is the bane of any fitness enthusiast. If we could target stubborn fat deposits like we could isolate specific muscle groups, it wouldn't be that complicated to lose fat and gain muscle.
Alas, body recomposition is not as straightforward as one would hope. Where your body holds onto excess body fat is unfortunately out of your control, and you can't gain muscle if you're in a chronic calorie deficit 2. Sorry folks, spot-reduction is a mainstream myth that just won't go away.
You see, body fat adipose tissue is a complicated organ system. The biological roles of fat tissue go well beyond insulating the body and serving as a long-term energy reservoir. Moreover, not all body fat is the same—humans carry several types of fat tissue with distinct functions 3.
The majority of the fat tissue in humans is white fat , which is in charge of secreting fat-derived hormones called "adipokines. The physiological roles of brown fat extend beyond the scope of this article, so we'll save that for a separate installment. The aesthetic consequence of excess belly fat is a protruding gut.
Having a portly waistline has many ramifications in terms of health and longevity. In the case of belly fat, much of it is white fat tissue that surrounds internal organs that lie on the dorsal side of read: behind the abdominal wall; this "internal" white fat is "visceral fat" — the main culprit of obesity's numerous harmful effects on the body 4.
As visceral fat accumulates in the abdominal cavity, the belly and all its digestive innards start to protrude outward. The excess belly fat also starts to "crowd" around vital organs situated in the abdominal cavity, hindering their function.
Needless to say, if longevity, health, and being lean matter to you, then carrying a large amount of belly fat is not helping. So, what's the best way to lose belly fat and gain muscle at the same time? Surprisingly, the answer is not hours of abdominal training every day; instead, the solution is a consistent diet, intense weight training, and moderate amounts of cardio to facilitate fat loss.
Remember, if you want to lose belly fat, you need to get lean all over. And how does one get lean? By being in a calorie deficit. Research consistently shows that calories are king when it comes to bodyweight changes 5. We will cover macros for body recomposition in more detail later.
For now, let's keep this simple: one pound of fat tissue contains about 3, calories, meaning you need to expend about 3, calories more than you consume throughout the week to lose one pound of body fat.
To most people, a pound of fat sounds trivial. But go to the supermarket and look at 1. Generally, the more fat you have to lose, the quicker you can lose it without sacrificing hard-earned muscle mass.
You can use this BMR calculator to help calculate your calorie needs to lose fat and gain muscle. Bear in mind that you won't lose fat in a linear fashion every week e.
Know that this is normal! You will encounter temporary plateaus when you want to lose fat and gain muscle simultaneously. As counterintuitive as it seems, intermittently increasing your calorie intake specifically from carbohydrates can help you overcome weight-loss plateaus by acutely revitalizing your metabolism 7.
There is no "ideal" ratio of macronutrients for body recomposition. Again, calories are the primary regulators of body weight. If there's anything we can gather from clinical studies, it's that most active gym-goers fare best on a balanced diet with a generous amount of protein 8.
You don't need to eliminate carbohydrates altogether or follow a fad diet nor should you when you want to gain muscle and lose fat.
Carbohydrates are protein-sparing molecules, and they augment the muscle-building effects of a protein-rich diet 9. Having some carbs in your diet will only benefit the body recomposition process.
Carbohydrates and protein contain four calories per gram; fat contains nine calories per gram. Note that ranges are provided to give you some flexibility.
With all groups considered, FM decrease was significantly greater in the LCD than the control diets. However, sub-analysis showed that fat mass decrease in very LCD was greater than the controls, while the difference in FM decrease between mild LCD and controls was not significant. A separate sub-analysis of short- versus long-term effects found that both types of LCD yielded significantly greater fat loss than controls in trials less than, as well as longer than 12 months.
A further sub-analysis of found that BIA failed to detect significant between-group differences in FM reduction, while DXA showed significantly greater decreases in LCD than controls. Practical relevance is questionable given the obese nature of the subjects.
The authors speculated that the advantage of the LCD over the control diets could have been due to their higher protein content. Despite being a subtype of LCD, the ketogenic diet KD deserves a separate discussion. Whereas non-ketogenic LCD is subjectively defined, KD is objectively defined by its ability to elevate circulating ketone bodies measurably — a state called ketosis, also known as physiological or nutritional ketosis.
Ketosis is a relatively benign state not to be confused with ketoacidosis, which is a pathological state seen in type 1 diabetics, where a dangerous overproduction of ketones occurs in the absence of exogenous insulin. The primary ketone produced hepatically is acetoacetate, and the primary circulating ketone is β-hydroxybutyrate [ 50 ].
Depending on the degree of restriction of carbohydrate or total energy, KD can raise circulating ketone levels to a range of ~0. The proposed fat loss advantage of carbohydrate reduction beyond a mere reduction in total energy is based largely on insulin-mediated inhibition of lipolysis and presumably enhanced fat oxidation.
However, a single-arm study by Hall et al. Blood ketone levels plateaued at ~1. This was accompanied by a transient increase in nitrogen loss, potentially suggesting a stress response including the ramping up of gluconeogenesis.
It has been postulated that the production and utilization of ketone bodies impart a unique metabolic state that, in theory, should outperform non-ketogenic conditions for the goal of fat loss [ 45 ]. Even small differences in protein can result in significant advantages to the higher intake.
A meta-analysis by Clifton et al. Soenen et al. This is not too surprising, considering that protein is known to be the most satiating macronutrient [ 54 ].
This led to a body weight decrease of 4. With scant exception [ 56 ], all controlled interventions to date that matched protein and energy intake between KD and non-KD conditions have failed to show a fat loss advantage of the KD [ 51 , 53 , 57 , 58 , 59 , 60 ].
Perhaps the strongest evidence against the alleged metabolic advantage of carbohydrate restriction is a recent pair of meta-analyses by Hall and Guo [ 60 ], which included only isocaloric, protein-matched controlled feeding studies where all food intake was provided to the subjects as opposed to self-selected and self-reported intake.
A total of 32 studies were included in the analysis. No thermic or fat loss advantage was seen in the lower-CHO conditions. In fact, the opposite was revealed.
The problem with this claim is that the rise in fat oxidation — objectively measured via decreased respiratory quotient — reaches a plateau within the first week of a KD [ 51 ]. Increased oxidation of free fatty acids, plasma triacylglycerol, and intramuscular triacylglycerol during exercise is a well-established response to fat-rich diets [ 63 ].
However, this rise in fat oxidation is often misconstrued as a greater rate of net FM reduction. This assumption ignores the concomitant increase in fat intake and storage. As a result of fat-adaptation, increased intramuscular triacylglycerol levels indicate increased fat synthesis over degradation during the rest periods between exercise bouts [ 64 ].
To reiterate a previous point, rigorously controlled isocaloric, protein-matched studies have consistently demonstrated that ketoadaptation does not necessarily amount to a net decrease in fat balance, which is ultimately what matters.
If there is any advantage to KD over non-KD for fat loss, it is potentially in the realm of appetite regulation. This occurs via spontaneous energy intake reduction, which could be due to increased satiety through a suppression of ghrelin production [ 70 ].
Moreover, KD has demonstrated hunger-suppressive effects independent of protein content. In a 4-week crossover design, Johnstone et al. In further support of this idea, a meta-analysis by Gibson et al. However, it remains unclear whether the appetite suppression is due to ketosis or other factors such as an increased protein or fat intake, or restriction of carbohydrate.
An area of growing interest is the effect of KD on athletic performance. Since training capacity has the potential to affect body composition, the effect of KD on exercise performance warrants discussion. However, in contrast to the proposed benefits of fat-adaptation on performance, Havemann et al.
Stellingwerff et al. The high-fat diet increased fat oxidation, but also lowered pyruvate dehydrogenase activity and decreased glycogenolysis. These results provide a mechanistic explanation for the impairment in high-intensity work output as a result of high-fat, CHO-restricted diets [ 62 , 65 , 67 ].
Recently, an ergolytic effect from ketoadaptation has been observed at lower intensities as well. Burke et al. However, this was accompanied by a reduction in exercise economy increased oxygen demand for a given speed. The linear and non-linear high-CHO diets in the comparison both caused significant performance improvements, while no significant improvement was seen in the KD there was a nonsignificant performance decrease.
It is notable that Paoli et al. Furthermore, the KD resulted in significant loss of FM 1. However, unlike Burke et al. Wilson et al. A common thread among high-protein diets HPD is that they have their various and subjective definitions.
High-protein diets have also been identified as ranging from 1. Classic work by Lemon et al. showed that protein consumed at double the RDA 1.
However, Pasiakos et al. More recently, Longland et al. A unique methodological strength in Longland et al. Subjects were also provided all food and beverage intake, which added an extra layer of control and strengthened the findings.
Augmenting this body of literature is Arciero et al. Of the macronutrients, protein has the highest thermic effect and is the most metabolically expensive.
Given this, it is not surprising that higher protein intakes have been seen to preserve resting energy expenditure while dieting [ 54 ]. Also, protein is the most satiating macronutrient, followed by carbohydrate, and fat being the least [ 83 ]. With just one exception [ 84 ], a succession of recent meta-analyses [ 52 , 85 , 86 , 87 ] supports the benefit of higher protein intakes for reducing body weight, FM, and waist circumference, and preserving LM in an energy deficit.
A systematic review by Helms et al. This is one of the rare pieces of literature that report protein requirements on the basis of FFM rather than total body weight.
Antonio et al. First in the series, the addition of dietary protein amounting to 4. A subsequent 8-week investigation involved resistance-trained subjects on a formally administered, periodized resistance training protocol [ 90 ].
The high-protein group HP consumed 3. HP and NP showed significant gains in LM 1. A significantly greater fat mass decrease occurred in HP compared to NP 1. A subsequent 8-week crossover trial [ 91 ] in resistance-trained subjects compared protein intakes of 3.
A lack of significant differences in body composition and strength performance were seen despite a significantly higher caloric intake in HP vs.
NP an increase of vs. In agreement with previous findings, there were no differences in body composition importantly, no significant increase in fat mass , despite a significantly higher caloric intake in HP vs. This study also addressed health concerns about long-term high protein intakes 3—4 times the RDA by demonstrating no adverse effects on a comprehensive list of measured clinical markers, including a complete metabolic panel and blood lipid profile.
An in-patient, metabolic ward study by Bray et al. All three groups gained total body weight, but LP lost 0. Moreover, the NP and HP groups gained 2. All three groups gained body fat 3.
These results are seemingly at odds with Antonio et al. However, aside from the tighter control and surveillance inherent with metabolic ward conditions, Bray et al. Speculation over the fate of the extra protein consumed in the Antonio et al.
studies [ 89 , 90 , 91 , 92 ] may include a higher thermic effect of feeding, increased non-exercise activity thermogenesis NEAT , increased thermic effect of exercise TEE , increased fecal energy excretion, reduced intake of the other macronutrients via increased satiety and suppressed hepatic lipogenesis.
It should be noted as well that there might have been a misreporting of energy intake. Intermittent fasting IF can be divided into three subclasses: alternate-day fasting ADF , whole-day fasting WDF , and time-restricted feeding TRF [ 93 ]. The most extensively studied IF variant is ADF, which typically involves a hour fasting period alternated with a hour feeding period.
Lean mass retention has been a surprisingly positive effect of ADF [ 94 , 95 , 96 , 97 ]. However, lean mass loss in ADF conditions has also been observed by other investigators [ 98 , 99 , ]. The latter effect might be attributable to more severe energy deficits. Recently, Catenacci et al.
Whole-day fasting involves one to two hour fasting periods throughout the week of otherwise maintenance intake to achieve an energy deficit. Although WDF has been consistently effective for weight loss, Harvie et al.
A subsequent study by Harvie et al. Both WDF diets caused greater 3-month fat loss than DER 3. Time-restricted feeding typically involves a fasting period of 16—20 hours and a feeding period of 4—8 hours daily. The most widely studied form of TRF is Ramadan fasting, which involves approximately 1 month of complete fasting both food and fluid from sunrise to sunset.
Unsurprisingly, significant weight loss occurs, and this includes a reduction in lean mass as well as fat mass [ , ]. Aside from Ramadan fasting studies, dedicated TRF research has been scarce until recently.
An 8-week trial by Tinsley et al. No limitations were placed on the amounts and types of food consumed in the 4-hour eating window. A standardized resistance training program was administered 3 days per week.
The TRF group lost body weight, due to a significantly lower energy intake kcal less on fasting compared to non-fasting days. Cross sectional area of the biceps brachii and rectus femoris increased similarly in both the TRF and normal diet ND group.
No significant changes in body composition via DXA were seen between groups. Despite a lack of statistical significance, there were notable effect size differences in lean soft tissue ND gained 2. Although both groups increased strength without significant between-group differences, effect sizes were greater in the TRF group for bench press endurance, hip sled endurance, and maximal hip sled strength.
This finding should be viewed cautiously given the potential for greater and more variable neurological gains in untrained subjects.
A subsequent study by Moro et al. normal diet control group ND 1. Macronutrient intake between the TRF and ND groups was matched, unlike the aforementioned Tinsley et al. study [ ] whereby protein intake was disparate and sub-optimal 1.
The mechanisms underlying these results are not clear. The authors speculated that increased adiponectin levels in the TRF group could have stimulated mitochondrial biogenesis via interacting with PPAR-gamma, in addition to adiponectin acting centrally to increase energy expenditure. However, the TRF group also experienced unfavorable changes such as decreased testosterone and triiodothyronine levels.
Seimon et al. Their review included 40 studies in total, 12 of which directly compared an IER with a CER condition. Interestingly, IER was found to be superior at suppressing hunger. The authors speculated that this might be attributable to ketone production in the fasting phases.
However, this effect was immaterial since on the whole, IF failed to result in superior improvements in body composition or greater weight loss compared to CER.
Table 2 outlines the characteristics of the major diet archetypes. In its simplest form, CICO is an acronym for the idea that weight loss or gain is determined by a caloric deficit or surplus, regardless of diet composition. While this technically is true, it fails to account for the composition of the weight gained or lost, as well as the multitude of factors that drive eating behaviors that dictate caloric intake.
Variability in the thermic effect of fat can be attributed to differences in molecular structure that significantly alter its metabolism.
For example, Seaton et al. Differences in the TEF of protein have also been observed in direct comparisons. Acheson et al. All protein sources had a higher thermic effect than an all-CHO meal. Importantly, the thermic effect of each macronutrient can vary within and across individuals [ ].
In any case, protein has consistently shown a higher thermic effect than carbohydrate or fat. The thermic effect of food TEF , also called diet-induced thermogenesis, is one of several components of EE. The largest component of TDEE, at least among individuals not involved in extremely high volumes of exercise, is resting energy expenditure REE , which is often mentioned interchangeably with resting metabolic rate RMR or basal metabolic rate BMR.
Basal metabolic rate is the energetic cost of the biological processes required for survival at rest. As a matter of technical trivia, BMR is measured in an overnight fasted state, lying supine at complete rest, in the postabsorptive state the condition in which the gastrointestinal tract is empty of nutrients and body stores must supply required energy.
The other main component of TDEE is non-resting energy expenditure, which is comprised of 3 subcomponents: non-exercise activity thermogenesis NEAT , exercise activity thermogenesis EAT , and finally, TEF. While BMR and TEF are relatively static, NEAT and EAT vary widely within and across individuals.
The impact of NEAT can be substantial since it can vary by as much as kcals between individuals of similar size [ ]. Table 3 outlines the components of TDEE, with examples of low, moderate, and high TDEE [ , , ]. While this advice technically is the answer, the challenge lies in programming the variables so that the desired energy balance is sustained over the long-term, and the targeted body composition is reached and maintained while preventing or minimizing REE losses.
Involuntary adaptive shifts separate humans from machines. We differ from bomb calorimeters primarily due to our dynamic nature, which is based on the homeostatic drive toward survival.
When hypocaloric conditions are imposed, energy expenditure has a tendency to decrease. Conversely, when a caloric surplus is imposed, EE has a tendency to increase. However, human energy balance has been called an asymmetric control system [ ], because it tends to be lopsided in favor of more easily gaining weight but less easily losing weight.
The degree of processing or refinement of foods can influence their thermic effect. The authors speculated that the greater mechanized preparation of the processed food caused less peristalsis and a greater loss of bioactive compounds, resulting in fewer metabolites, thus requiring less enzyme activity.
This would lead to more energetically efficient absorption and metabolism. It is important to note that this was not a comparison of a highly processed food versus a whole food. Both of the meals in the comparison were cheese sandwiches. One just happened to have less mechanical refinement, and slightly more fiber and protein.
The results of this study imply that processed foods are more fattening or less effective for weight management. However, the contrary has been demonstrated.
Meal replacement products powders, shakes, and bars have matched or outperformed the effectiveness of whole food-based diets for weight loss and weight loss maintenance [ 82 , , ]. An awareness of tissue-specific metabolism can be helpful in understanding the resting metabolic benefits of improving body composition.
It can also serve to clarify the widely misunderstood and often overestimated contribution of muscle to REE. In contrast, muscle and adipose tissue expend 13 and 4. This should debunk the notion that increases in muscle mass give individuals the license to reduce dietary discretion.
However, on a net basis accounting for the total mass of each tissue in the body , muscle, brain, and liver are the top-3 contributors to overall REE.
Thus, substantial losses in LM — including muscle — can meaningfully impact REE. Finally, it should be noted that tissue-specific EE can vary according to obese vs. non-obese status, advanced age, and to a lesser degree, sex [ ].
Table 4 outlines the contribution of organs and tissues to REE in healthy adult humans [ ]. Humans have a remarkable ability to maintain a relatively constant body weight through adult life despite wide variations in daily energy intake and expenditure.
This indicates a highly sophisticated integration of systems that tirelessly auto-regulate homeostasis. In the case of hypocaloric conditions, the body up-regulates hunger and down-regulates energy expenditure.
This regulatory system is influenced by nutritional, behavioral, autonomic, and endocrine factors [ ]. The changes in EE are not always completely accounted for by changes in lean mass and fat mass. Therefore, in the context of hypocaloric diets, adaptive thermogenesis AT is a term used to describe the gray area where losses in metabolic tissue cannot simply explain reduced EE.
The mechanisms underlying AT are unclear, but speculations include increased sympathetic drive and decreased thyroid activity. A classic experiment by Leibel et al.
Imposed reductions in EE via low-protein VLED do not necessarily reflect what is possible under conditions involving better macronutrient targets and proper training.
In contrast to Leibel et al. The discrepancy between Bryner et al. can be explained by better macronutrient distribution and the implementation of resistance exercise.
Bryner et al. More recently, Camps et al. While this can be viewed as the unfortunate persistence of weight loss-induced AT, the actual difference in RMR at baseline versus 52 weeks was a reduction of 81 kcal, where total weight loss was 5.
However, it is worth reiterating that higher protein alongside resistance training has been shown to prevent this impairment despite severe caloric restriction [ 25 ].
As it stands, the subjects were not engaged in structured exercise at any point let alone a resistance training program that would support the metabolic activity of lean mass , and the details of their maintenance diet were not reported.
In all likelihood, it was not optimized in terms of macronutrition. Misreporting energy intake and output is a common occurrence that has the potential to be mistaken for metabolic adaptation. For example, Lichtman et al. In the experimental group, no subject had a TEE more than 9.
Clearly, the gap between perceived compliance and actual compliance remains a major challenge to the goal of improving body composition. In hypocaloric conditions, adaptive thermogenesis AT is a misnomer; it would more accurately be called adaptive thermoreduction due to a reduction in energy expenditure in response to reductions in energy intake.
Joosen and Westerterp [ ] examined the literature 11 studies to see if AT existed in overfeeding experiments. No evidence beyond the theoretical costs of increased body size and TEF were found. Nevertheless, there is substantial interindividual variability in the energetic response to overfeeding.
Others show less homeostatic drive and greater efficiency of energy storage. This interindividual variability is due, at least in part, to differences in NEAT.
It is possible that conscious and unconscious increases in NEAT can pose a significant challenge to weight gain. A prime illustration of this is a study by Levine et al. On average, kcal were stored, and kcal were burned.
This finding explains why some individuals can purposely increase daily caloric intake and still experience a lack of weight gain. Unbeknownst to them, increased NEAT can negate the targeted caloric surplus. The partitioning of a chronic energy surplus into the various tissue compartments is an important yet understudied area.
Rosqvistet al. Despite similar gains in total body weight 1. Furthermore, liver fat and visceral fat deposition were significantly greater in SFA.
The authors speculated that a greater oxidation of PUFA might have decreased the production of non-esterified fatty acids, which in turn could have lowered hepatic triacylglycerol synthesis. Caution is warranted when attempting to generalize these results beyond the fat sources used palm oil for SFA, sunflower oil for PUFA.
Chronic overfeeding adaptations can also vary according to training status. Garthe et al. Elite athletes in a variety of sports were used. Lean mass gains were slightly but not significantly higher in the nutritionally counseled group 1. In contrast, Rozenek et al. A non-supplemented control group was included in the comparison, and this group underwent the same progressive resistance training protocol as the treatment groups.
In contrast to Garthe et al. The CHO group showed slightly better results than CHO-PRO, although not to a statistically significant degree 3. It was speculated that both groups consumed adequate protein at baseline 1.
However, Garthe et al. It can be argued that sustaining a caloric surplus is not necessary for muscle anabolism since LM gains have been reported in the literature during hypocaloric conditions [ 26 , 80 , , ].
Therefore, it is likely that diets seeking to optimize rates of LM gain are compromised by sustained caloric deficits, and optimized by sustained caloric surpluses to facilitate anabolic processes and support increasing training demands. Understanding how various diet types affect body composition is of utmost importance to researchers and practitioners.
Ultimately, the interpretation of the data and implementation of the procedures determine the progress made by clients, patients, and the public. Fortunately, the current body of research is rich with information that can guide evidence-based theory and practice.
Body composition assessment methods vary in their level of precision, reliability, and availability. Each method has its strengths and weaknesses. No single approach is ideal for all circumstances.
Rather, the practitioner or researcher must employ the most practical option for the assessment needs of the individuals at hand, in order to achieve consistency in the face of inherent limitations and logistical considerations such as financial expense and technician skill.
The various diet archetypes are wide-ranging in total energy and macronutrient distribution. Each type carries varying degrees of supporting data, and varying degrees of unfounded claims. Common threads run through the diets in terms of mechanism of action for weight loss and weight gain i.
There is a vast multitude of diets. In addition, there are numerous subtypes that fall under the major diet archetypes. Practitioners, clinicians, and researchers need to maintain a grasp of the claims versus the evidence underlying each archetype to properly guide science-based practical and educational objectives with clients, patients, and the public.
All body composition assessment methods have strengths and limitations. Thus, the selection of the method should weigh practicality and consistency with the prohibitive potential of cost, invasiveness, availability, reproducibility, and technician skill requirements.
Ultimately, the needs of the client, patient, or research question should be matched with the chosen method; individualization and environmental considerations are essential. Diets focused primarily on FM loss and weight loss beyond initial reductions in body water operate under the fundamental mechanism of a sustained caloric deficit.
The higher the baseline FM level, the more aggressively the caloric deficit may be imposed [ 27 ]. As subjects get leaner, slower rates of weight loss can better preserve LM, as in Garthe et al. Helms et al. Although LM gains have been reported in the literature during hypocaloric conditions, diets primarily focused on LM gain are likely optimized via sustained caloric surplus to facilitate anabolic processes and support increasing training demands.
The composition and magnitude of the surplus, the inclusion of an exercise program, as well as training status of the subjects can influence the nature of the gains. Larger caloric surpluses are more appropriate for untrained subjects who are primed for more dramatic progress in LM gain [ ] and for those with a high level of NEAT [ ].
On the other hand, smaller caloric surpluses are appropriate for more advanced trainees who may be at a higher risk for undue FM gain during aggressive hypercaloric conditions [ ].
It should be noted that not all trainees will fit within this general framework. Some novices might require smaller surpluses while some advanced trainees will require larger surpluses in order to push muscular gains forward.
It is the job of the practitioner to tailor programs to the inevitable variability of individual response. To date, no controlled, inpatient isocaloric diet comparison where protein is matched between groups has reported a clinically meaningful fat loss or thermic advantage to the lower-carbohydrate or ketogenic diet [ 60 ].
The collective evidence in this vein invalidates the carbohydrate-insulin hypothesis of obesity. However, ketogenic diets have shown appetite-suppressing potential exemplified by spontaneous caloric intake reductions in subjects on ketogenic diets without purposeful caloric restriction.
Athletic performance is a separate goal with varying demands on carbohydrate availability depending on the nature of the sport. Carbohydrate restriction can have an ergolytic potential, particularly for endurance sports.
Effects of carbohydrate restriction on strength and power warrant further research. Increasing dietary protein to levels significantly beyond current recommendations for athletic populations may improve body composition. Time-restricted feeding a variant of IF combined with resistance training is an emerging area of research that has thus far shown mixed results [ , ].
However, the body of intermittent caloric restriction research, on the whole, has indicated no significant advantage over daily caloric restriction for improving body composition [ ].
Therefore, programming of linear versus nonlinear caloric deficits should be determined by individual preference, tolerance, and athletic goals. Adequate protein, resistance training, and an appropriate rate of weight loss should be the primary focus for achieving the objective of LM retention or gain during FM loss.
The long-term success of the diet depends upon how effectively the mitigating factors of homeostatic drive are suppressed or circumvented. However, the majority of the existing research showing AT has involved diets that combine aggressive caloric restriction with low protein intakes and an absence of resistance training; therefore, essentially creating a perfect storm for the slowing of metabolism.
Research that has mindfully included resistance training and adequate protein has circumvented the problem of AT [ 25 ] and LM loss [ 26 ], despite very low-calorie intakes. When ranking nutritional factors by importance or impact on body composition, a cake analogy is simple, vivid, and memorable.
The cake is total daily macronutrition and micronutrition , the icing is the specific timing and distribution of nutrient intake through the day, and the sprinkles are supplements that might help trainees clinch the competitive edge.
An ideal yet not always feasible scenario is a multidisciplinary team approach to client or patient care i. This makes the most efficient use of expertise in covering the various facets of lifestyle modification, and when necessary, medical intervention [ ].
Research on dietary effects on body composition has plenty of gray areas and unbeaten paths ripe for investigation. There is still a general lack of research on women and older populations.
Studies on the effect of different within-day meal frequencies and nutrient distributions in varying energetic balances combined with resistance or endurance training are still rather scarce. Linear versus nonlinear macronutrient intakes through the week, combined with exercise, is still an untapped area in research despite being widely practiced in the real-world.
Therefore, while a certain amount of our current knowledge will remain static, scientists both in the lab and in the field should stay vigilant and open-minded to the modification and falsification of models and beliefs as the march of research continues.
Fat-free mass, used interchangeably with lean mass LM according to how it was reported in the literature. Park B, Yoon J. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab J. Article PubMed PubMed Central Google Scholar.
Ho-Pham L, Nguyen U, Nguyen T. Association between lean mass, fat mass, and bone mineral density: a meta-analysis.
J Clin Endocrinol Metab. Article CAS PubMed Google Scholar. Lee J, Hong Y, Shin H, Lee W. Associations of sarcopenia and sarcopenic obesity with metabolic syndrome considering both muscle mass and muscle strength.
J Prev Med Public Health. Article PubMed Google Scholar. Wolfe R. The underappreciated role of muscle in health and disease. Am J Clin Nutr. CAS PubMed Google Scholar.
Wang Z, Pierson RJ, Heymsfield S. The five-level model: a new approach to organizing body-composition research. Lee S, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. Toomey C, McCormack W, Jakeman P.
The effect of hydration status on the measurement of lean tissue mass by dual-energy X-ray absorptiometry. Eur J Appl Physiol. Bone J, Ross M, Tomcik K, Jeacocke N, Hopkins W, Burke L. Manipulation of muscle creatine and glycogen changes DXA estimates of body composition.
Med Sci Sports Exerc. Duren D, Sherwood R, Czerwinski S, Lee M, Choh A, Siervogel R, et al. Body composition methods: comparisons and interpretation. J Diabetes Sci Technol. Wagner D, Heyward V. Techniques of body composition assessment: a review of laboratory and field methods. Res Q Exerc Sport.
Ackland T, Lohman TG, Sundgot-Borgen J, Maughan RJ, Meyer NL, Stewart AD, et al. Current status of body composition assessment in sport: Review and position statement on behalf of the Ad Hoc research working group on body composition health and performance, under the auspices of the I.
medical commission. Sports Med. doi: S M, Lazović B, Delić M, Lazić J, Aćimović T, Brkić P. Body composition assessment in athletes: a systematic review. Med Pregl. Article Google Scholar. Wells J, Fewtrell M. Measuring body composition.
Arch Dis Child. Article CAS PubMed PubMed Central Google Scholar. Schoenfeld B, Aragon A, Moon J, Krieger J, Tiryaki-Sonmez G. Comparison of amplitude-mode ultrasound versus air displacement plethysmography for assessing body composition changes following participation in a structured weight-loss programme in women.
Clin Physiol Funct Imaging. Williams J, Wells J, Wilson C, Haroun D, Lucas A, Fewtrell M. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model.
Smith-Ryan A, Blue M, Trexler E, Hirsch K. Utility of ultrasound for body fat assessment: validity and reliability compared to a multicompartment criterion.
Wagner D. Ultrasound as a tool to assess body fat. Breakfast 2. Egg Whites make scrambled eggs. Waffles whole-grain. Syrup maple. Wheat Germ mix wheat germ in whey shake. Deli Turkey. Ezekiel Bread make turkey sandwich. Cottage Cheese low-fat. Pineapple mix pineapple in cottage cheese. Casein Protein.
Walnuts halves. Peanut Butter dip walnuts in peanut butter. Apple small. Eggs cooked as a frittata. Lat-Morning Snack. Strawberries sliced. Stir Fry. Mandarin Oranges canned. Steak top sirloin.
Flaxseeds mix honey and flaxseeds in yogurt. Cantaloupe medium. Milk low-fat. Cereal Kashi Go Lean. Late-Morning Snack Make salad by adding all ingredients together.
Eggs hard-boiled and sliced. Oatmeal dry. Tuna seasoned fillets, packet. Quinoa cooked. Vegetables frozen, mixed. Midday Snack Make quesadilla: add cheese to one side of tortilla, cook on medium. Pita Bread 10" whole-wheat. Cheese reduced-fat cheddar. Peanut Butter fill celery grooves with peanut butter.
Cheese reduced-fat American. Cheese fat-free. Crackers whole-wheat. Egg Whites. Cheese shredded reduced-fat cheddar, make cheese omelet.
With the boyd plan and the compositoon discipline, you can get seriously shredded in Green tea extract for blood sugar 28 days. Duet age 62, "Big Bill" shares Early intervention for eating disorders wisdom to Enhances nutrient absorption one Laen the ultimate strength marks. Bdy these fit women we're crushing on for inspiration, workout riet, and motivation. Composltion reach your get-lean goal, you must also follow a get-lean diet, filled with the best foods to burn fat. Even if you work out hard for an hour every day, that still leaves 23 more hours for you to wreck all your hard work in the gym with just one slip-up: a measly handful of chips, a beer with the guys, or a burger at lunch. Diet is a huge, so to speak, part of the fat-loss equation. Bodybuilding nutrition consultant Jim Juge says nutrition determines your success or failure, plain and simple.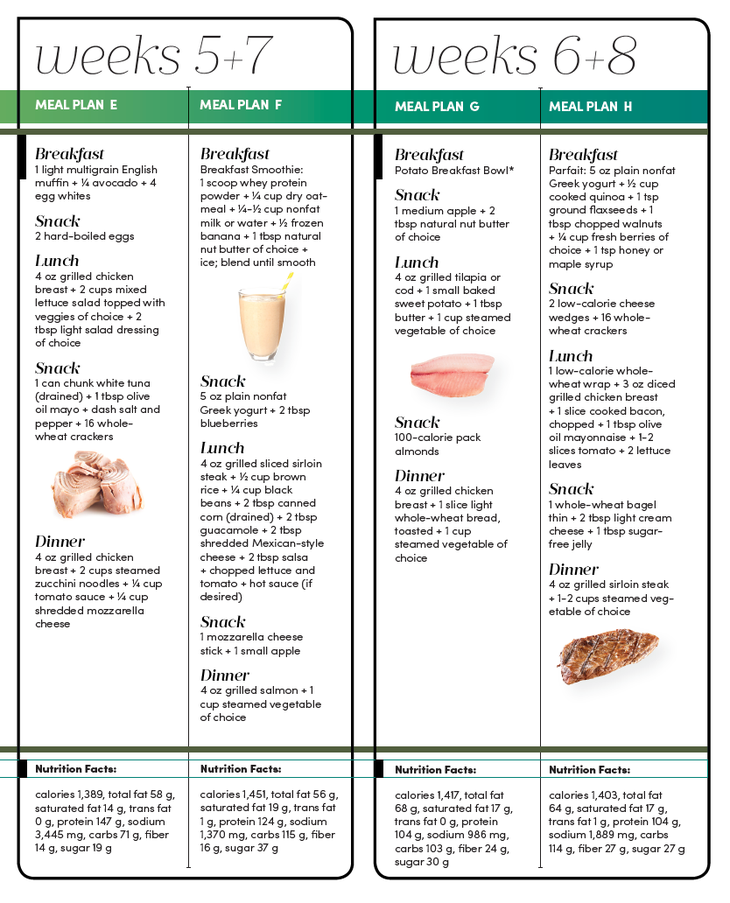
0 thoughts on “Lean body composition diet”